Abstract
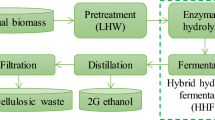
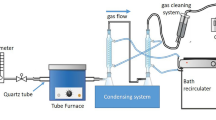
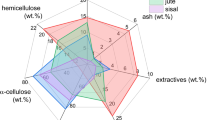
Lignocellulosic feedstocks are utilized for the production of fuel ethanol and butanol through dilute acid/enzymatic hydrolysis and fermentation. Hydrolysis residue, a major by-product of biomass hydrolysis, is rich in recalcitrant carbon as majority of cellulosic and hemicellulosic components are released during pretreatment. With the intention of their effective utilization, hydrolysis residues from forestry (pinewood), energy crop system (timothy grass), and agriculture (wheat straw) were pyrolysed in a fixed-bed reactor at 600 °C with slow heating rate of 5 °C/min for 4 h. In order to understand the product (biochar, bio-oil, and gases) properties and advocate their energy and environmental values, chemical characterizations such as carbon–hydrogen–nitrogen–sulfur analysis, inductively coupled plasma-mass spectrometry, pH, electrical conductivity, scanning electron microscopy, porosity analysis, thermogravimetric analysis, X-ray diffraction, Fourier transform infrared (FTIR) and Raman spectroscopy, nuclear magnetic resonance (NMR) and gas chromatography–mass spectrometry (GC-MS) were employed. The yield of biochar, bio-oil and gases was 38.9–41.7, 18.6–22.3, and 24.9–28.8 wt%, respectively. The high pH and electrical conductivity of biochars with substantial amounts of Na, Mg, K, and Ca indicated their alkaline and saline nature, which would necessitate proper agronomical soil applications. Variable intensities of C–C, C–H, C–O, O–H, and C–N functional groups were detected in the FTIR spectra of residues, biochars, and bio-oils. Raman spectroscopy showed the development of graphite (1,580–1,610 cm−1) and defect (1,325–1,380 cm−1) carbon structures in biochars. 1H NMR of bio-oils indicated aromatics, olefinics, and aliphatics, whereas 13C NMR indicated carbonyls, aromatics, carbohydrates, alkyls, methoxy, and hydroxy carbon. GC studies of pyrolysis gases identified chiefly H2 and CO with traces of CH4, CO2, and C2+ components.









Similar content being viewed by others
References
Hu F, Ragauskas A (2012) Pretreatment and lignocellulosic chemistry. Bioenerg Res 5:1043–1066. doi:10.1007/s12155-012-9208-0
Mabee WE, Saddler JN (2010) Bioethanol from lignocellulosics: status and perspectives in Canada. Bioresour Technol 101:4806–4813. doi:10.1016/j.biortech.2009.10.098
USEIA, United States Energy Information Administration (2013) Independent statistics and analysis. http://www.eia.gov/countries. Accessed 14 February 2013.
Girio FM, Fonseca C, Carvalheiro F, Duarte LC, Marques S, Bogel-Lukasik R (2010) Hemicelluloses for fuel ethanol: a review. Bioresour Technol 101:4775–4800. doi:10.1016/j.biortech.2010.01.088
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729. doi:10.1021/ie801542g
Huang Y, Wei Z, Yin X, Wu C (2012) Pyrolytic characteristics of biomass acid hydrolysis residue rich in lignin. Bioresour Technol 103:470–476. doi:10.1016/j.biortech.2011.10.027
Wang Z, Lin W, Song W, Wu X (2012) Pyrolysis of the lignocellulose fermentation residue by fixed-bed micro reactor. Energy 43:301–305. doi:10.1016/j.energy.2012.04.026
Kim S, Dale EB (2004) Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenerg 26:361–375. doi:10.1016/j.biombioe.2003.08.002
Kern S, Halwachs M, Kampichler G, Pfeifer C, Proll T, Hofbauer H (2012) Rotary kiln pyrolysis of straw and fermentation residues in a 3 MW pilot plant – influence of pyrolysis temperature on pyrolysis product performance. J Anal Appl Pyrol 97:1–10. doi:10.1016/j.jaap.2012.05.006
Yao Y, Gao B, Inyang M, Zimmerman AR, Cao X, Pullammanappallil P, Yang L (2011) Biochar derived from anaerobically digested sugar beet tailings: characterization and phosphate removal potential. Bioresour Technol 102:6273–6278. doi:10.1016/j.biortech.2011.03.006
Nanda S, Mohanty P, Pant KK, Naik S, Kozinski JA, Dalai AK (2013) Characterization of North American lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels. Bioenerg Res 6:663–677. doi:10.1007/s12155-012-9281-4
Qureshi N, Saha BC, Dien B, Hector RE, Cotta MA (2010) Production of butanol (a biofuel) from agricultural residues: Part I – use of barley straw hydrolysate. Biomass Bioenerg 34:559–565. doi:10.1016/j.biombioe.2009.12.024
ASTM E871-82 (2006) Standard test method for moisture analysis of particulate wood fuels. ASTM International, Pennsylvania. doi: 10.1520/E0871-82R06
ASTM E1755-01 (2007) Standard test method for ash in biomass. ASTM International, Pennsylvania. doi: 10.1520/E1755-01R07
ASTM D3175-11 (2011) Standard method for volatile matter in the analysis sample of coal and coke. ASTM International, Pennsylvania. doi: 10.1520/D3175-11
ASTM D1762-84 (2007) Standard test method for chemical analysis of wood charcoal. ASTM International, Pennsylvania. doi: 10.1520/D1762-84R07
ASTM D3176-09 (2009) Standard practice for ultimate analysis of coal and coke. ASTM International, Pennsylvania. doi: 10.1520/D3176-09
ASTM D5373-08 (2008) Standard test methods for instrumental determination of carbon, hydrogen, and nitrogen in laboratory samples of coal. doi: 10.1520/D5373-08
USEPA, United States Environmental Protection Agency (2007) Method 3051A: microwave assisted acid digestion of sediments, sludges, soils, and oils. http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/3051a.pdf. Accessed 01 June 2013.
Anon JAR, Lopez FF, Castineiras JP, Ledo JP, Regueira LN (1995) Calorific values and flammability for forest wastes during the seasons of the year. Bioresour Technol 52:269–274. doi:10.1016/0960-8524(95)00034-C
Lv PM, Xiong ZH, Chang J, Wu CZ, Chen Y, Zhu ZX (2004) An experimental study on biomass air–steam gasification in a fluidized bed. Bioresour Technol 95:95–101. doi:10.1016/j.biortech.2004.02.003
Song W, Guo M (2012) Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J Anal Appl Pyrol 94:138–145. doi:10.1016/j.jaap.2011.11.018
Segal L, Creely JJ, Martin AE Jr, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-Ray diffractometer. Text Res J 29:786–794. doi:10.1177/004051755902901003
Duman G, Okutucu C, Ucar S, Stahl R, Yanik J (2011) The slow and fast pyrolysis of cherry seed. Bioresour Technol 102:1869–1878. doi:10.1016/j.biortech.2010.07.051
Obernberger I, Brunner T, Barnthaler G (2006) Chemical properties of solid biofuels – significance and impact. Biomass Bioenerg 30:973–982. doi:10.1016/j.biombioe.2006.06.011
Mattson WJ Jr (1980) Herbivory in relation to plant nitrogen content. Ann Rev Ecol Syst 11:119–161. doi:10.1146/annurev.es.11.110180.001003
Fu P, Hua S, Xiang J, Sun L, Su S, Wang J (2012) Evaluation of the porous structure development of chars from pyrolysis of rice straw: Effects of pyrolysis temperature and heating rate. J Anal Appl Pyrol 98:177–183. doi:10.1016/j.jaap.2012.08.005
Francioso O, Sanchez-Cortes S, Bonora S, Roldan ML, Certini G (2011) Structural characterization of charcoal size-fractions from a burnt Pinus pinea forest by FT-IR, Raman and surface-enhanced Raman spectroscopies. J Mol Struct 994:155–162. doi:10.1016/j.molstruc.2011.03.011
Lehmann J (2007) Bio-energy in the black. Front Ecol Environ 5:381–387. doi: 10.1890/1540-9295(2007)5[381:BITB]2.0.CO;2
Vassilev SV, Baxter D, Andersen LK, Vassileva CG (2013) An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 105:40–76. doi:10.1016/j.fuel.2012.09.041
Rhoades JD, Chanduvi F, Lesch S (1999) Soil salinity assessment: methods and interpretation of electrical conductivity measurements. FAO Irrigarion and Drainage Paper 57. Rome, Italy.
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572. doi:10.1016/j.plaphy.2004.05.009
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota – a review. Soil Biol Biochem 43:1812–1836. doi:10.1016/j.soilbio.2011.04.022
Azargohar R, Dalai AK (2008) Steam and KOH activation of bio-char: experimental and modeling studies. Microporous Mesoporous Mater 110:413–421. doi:10.1016/j.micromeso.2007.06.047
Tatarkova V, Hiller E, Vaculik M (2013) Impact of wheat straw biochar addition to soil on the sorption, leaching, dissipation of the herbicide (4-chloro-2-methylphenoxy)acetic acid and the growth of sunflower (Helianthus annuus L.). Ecotox Environ Safe 92:215–221. doi:10.1016/j.ecoenv.2013.02.005
Liu Z, Zhang F-S, Wu J (2010) Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 89:510–514. doi:10.1016/j.fuel.2009.08.042
Novak JM, Lima I, Xing B, Gaskin JW, Steiner C, Das KC, Ahmedna M, Rehrah D, Watts DW, Busscher WJ, Schomberg H (2009) Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann Environ Sci 2:195–206
Rivera-Utrilla J, Bautista-Toledo I, Ferro-Garcia MA, Moreno-Castilla C (2001) Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J Chem Technol Biotechnol 76:1209–1215. doi:10.1002/jctb.506
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18. doi:10.1007/s11104-010-0464-5
Werkelin J, Skrifvars BJ, Hupa M (2005) Ash-forming elements in four Scandinavian wood species. Part. 1. Summer harvest. Biomass Bioenerg 29:451–466. doi:10.1016/j.biombioe.2005.06.005
Vassilev SV, Baxter D, Andersen LK, Vassileva CG (2010) An overview of the chemical composition of biomass. Fuel 89:913–933. doi:10.1016/j.fuel.2009.10.022
Kim P, Johnson A, Edmunds CW, Radosevich M, Vogt F, Rials TG, Labbe N (2011) Surface functionality and carbon structures in lignocellulosic-derived biochars produced by fast pyrolysis. Energ Fuel 25:4693–4703. doi:10.1021/ef200915s
Das KC, Garcia-perez M, Bibens B, Melear N (2008) Slow pyrolysis of poultry litter and pine woody biomass: impact of chars and bio-oils on microbial growth. J Environ Sci Health Part A 43:714–724. doi:10.1080/10934520801959864
Jeguirim M, Trouve G (2009) Pyrolysis characteristics and kinetics of Arundo donax using thermogravimetric analysis. Bioresour Technol 100:4026–4031. doi:10.1016/j.biortech.2009.03.033
Vassilev SV, Baxter D, Andersen LK, Vassileva CG, Morgan TJ (2012) An overview of the organic and inorganic phase composition of biomass. Fuel 94:1–33. doi:10.1016/j.fuel.2011.09.030
Chen B, Zhou D, Zhu L (2008) Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol 42:5137–5143. doi:10.1021/es8002684
Siengchum T, Isenberg M, Chuang SSC (2013) Fast pyrolysis of coconut biomass – an FTIR study. Fuel 105:559–565. doi:10.1016/j.fuel.2012.09.039
Knicker H (2007) How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 85:91–118. doi:10.1007/s10533-007-9104-4
Ozcimen D, Ersoy-Mericboyu A (2010) Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials. Renew Energ 35:1319–1324. doi:10.1016/j.renene.2009.11.042
Li C, Knierim B, Manisseri C, Arora R, Scheller HV, Auer M, Vogel KP, Simmons BA, Singh S (2010) Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol 101:4900–4906. doi:10.1016/j.biortech.2009.10.066
Li XJ, Hayashi J, Li CZ (2006) FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 85:1700–1707. doi:10.1016/j.fuel.2006.03.008
Reich S, Thomsen C (2004) Raman spectroscopy of graphite. Phil Trans R Soc Lond A 362:2271–2288
Steiner C, Das KC, Melear N, Lakly D (2010) Reducing nitrogen loss during poultry litter composting using biochar. J Environ Qual 39:1236–1242. doi:10.2134/jeq2009.0337
Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agron 105:47–82. doi:10.1016/S0065-2113(10)05002-9
Spokas KA, Reicosky DC (2009) Impacts of sixteen different biochars on soil greenhouse gas production. Ann Environ Sci 3:179–193
Keown DM, Li X, Hayashi J, Li CZ (2008) Evolution of biomass char structure during oxidation in O2 as revealed with FT-Raman spectroscopy. Fuel Process Technol 89:1429–1435. doi:10.1016/j.fuproc.2008.07.002
Akin DE (2007) Grass lignocellulose: strategies to overcome recalcitrance. Appl Biochem Biotechnol 137–140:3–15. doi:10.1007/s12010-007-9035-5
Yong TLK, Matsumura Y (2012) Reaction kinetics of the lignin conversion in supercritical water. Ind Eng Chem Res 51:11975–11988. doi:10.1021/ie300921d
Pittman CU Jr, Mohan D, Eseyin A, Li Q, Ingram L, Hassan EBM, Mitchell B, Guo H, Steele PH (2012) Characterization of bio-oils produced from fast pyrolysis of corn stalks in an auger reactor. Energ Fuel 26:3816–3825. doi:10.1021/ef3003922
Henstra AM, Sipma J, Rinzema A, Stams AJM (2007) Microbiology of synthesis gas fermentation for biofuel production. Curr Opin Biotechnol 18:200–206. doi:10.1016/j.copbio.2007.03.008
Acknowledgments
The authors would like to thank Lassonde School of Engineering at York University and Saskatchewan Structural Sciences Centre at University of Saskatchewan for the research facilities. The funding support from Natural Sciences and Engineering Research Council of Canada (NSERC) and Canada Research Chair (CRC) program is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nanda, S., Azargohar, R., Kozinski, J.A. et al. Characteristic Studies on the Pyrolysis Products from Hydrolyzed Canadian Lignocellulosic Feedstocks. Bioenerg. Res. 7, 174–191 (2014). https://doi.org/10.1007/s12155-013-9359-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-013-9359-7