Abstract
Objectives
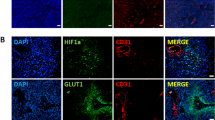
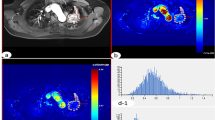
Hypoxia is a key element involved in the development and progression of tumors. HIF-1α may transiently induce and mediate the response to acute and severe hypoxia, while HIF-2α may induce a longer response and may control the response to chronic and moderate hypoxia. Hypoxia increases the cellular uptake of FDG. Therefore, HIF may play an important role in the process of the cellular uptake of FDG. The aim of this study was to compare HIF-1α/HIF-2α expression with FDG uptake, Glut-1 expression, and prognosis in the patients with lung adenocarcinoma and to investigate the role of HIF-1α/HIF-2α in the uptake of FDG in lung adenocarcinoma.
Methods
In the current work, we compared the immunohistochemical expression of HIF-1α and HIF-2α in surgical specimens of 44 patients with lung adenocarcinoma. The relationships between HIF-α expression and Glut-1 expression, FDG uptake, and clinicopathological factors, including prognosis, were analyzed.
Results
There was a marginal association between HIF-1α and HIF-2α expressions (P = 0.076). We found a significant correlation between HIF-2α expression and FDG uptake (P = 0.0001). HIF-1α expression showed a marginal association with FDG uptake (P = 0.066). FDG uptake correlated more significantly with HIF-2α expression than with HIF-1α expression. A significant correlation was noticed between Glut-1 expression and both HIF-1α and HIF-2α expressions (P = 0.005 and P = 0.003, respectively). Univariate analysis of disease-free survival demonstrated that FDG uptake and HIF-2α expression, but not HIF-1α expression, were related to recurrence (P < 0.0001).
Conclusion
FDG uptake correlated more significantly with HIF-2α expression than with HIF-1α expression, and both FDG uptake and HIF-2α expression, but not HIF-1α expression was correlated with post-operative recurrence in the patients with lung adenocarcinoma. These results suggest that both FDG uptake and HIF-2α expression may represent a more aggressive phenotype and that HIF-2α may play a more important role than HIF-1α in the uptake of FDG in lung adenocarcinoma.


Similar content being viewed by others
References
Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–43.
Dachs GU, Patterson AV, Firth JD, Ratcliffe PJ, Townsend KM, Stratford IJ, et al. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997;3:515–20.
Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:1172–80.
Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84.
Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–54.
Maxwell PH, Ratcliffe PJ. Oxygen sensors and angiogenesis. Semin Cell Dev Biol. 2002;13:29–37.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32.
Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, et al. HIF-2 alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–70.
Sato M, Tanaka T, Maemura K, Uchiyama T, Sato H, Maeno T, et al. The Pai-1 gene as a direct target of endothelial PAS domain protein-1 in adenocarcinoma A549 cells. Am J Respir Cell Mol Biol. 2004;31:209–15.
Pedersen MW, Holm S, Lund EL, Højgaard L, Kristjansen PE. Coregulation of glucose uptake and vascular endothelial growth factor (VEGF) in two small-cell lung cancer (SCLC) sublines in vivo and in vitro. Neoplasia. 2001;3:80–7.
Clavo AC, Brown RS, Wahl RL. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J Nucl Med. 1995;36:1625–32.
Guo J, Higashi K, Ueda Y, Oguchi M, Takegami T, Toga H, et al. Microvessel density: correlation with 18F-FDG uptake and prognostic impact in lung adenocarcinomas. J Nucl Med. 2006;47:419–25.
Guo J, Higashi K, Ueda Y, Ishigaki Y, Sakuma T, Oguchi M, et al. VEGF-A and its isoform VEGF121 mRNA expression measured by quantitative real-time RT-PCR: correlation with F-18 FDG uptake and aggressiveness of lung adenocarcinoma: preliminary study. Ann Nucl Med. 2011;25:29–36.
van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1α and GLUT-1 in non-small cell lung cancer. Eur J Cancer. 2007;43:1392–8.
Dooms C, Verbeken E, Stroobants S, Vansteenkiste J. Biological correlates of the maximum 18-fluoro-2-deoxy-glucose uptake on positron emission tomography in non-small cell lung carcinoma after induction chemotherapy. J Thorac Oncol. 2009;4:1221–5.
Kaira K, Endo M, Abe M, Nakagawa K, Ohde Y, Okumura T, et al. Biologic correlates of 18F-FDG uptake on PET in pulmonary pleomorphic carcinoma. Lung Cancer. 2011;71:144–50.
Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, et al. Expression of vascular endothelial growth factor (VEGF)-D and its receptor, VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin Cancer Res. 2003;9:1361–9.
Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–74.
Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, et al. Relation of hypoxia inducible factor 1α and 2α in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–90.
Wu XH, Qian C, Yuan K. Correlations of hypoxia-inducible factor-1α/hypoxia-inducible factor-2α expression with angiogenesis factors expression and prognosis in non-small cell lung cancer. Chin Med J. 2011;124:11–8.
Higashi K, Ito K, Hiramatsu Y, Ishikawa T, Sakuma T, Matsunari I, et al. 18F-FDG uptake by primary tumor as a predictor of intratumoral lymphatic vessel invasion and lymph node involvement in non-small cell lung cancer: analysis of a multicenter study. J Nucl Med. 2005;46:267–73.
Tsutani Y, Miyata Y, Misumi K, Ikeda T, Mimura T, Hihara J, et al. Difference in prognostic significance of maximum standardized uptake value on [18F]-fluoro-2-deoxyglucose positron emission tomography between adenocarcinoma and squamous cell carcinoma of the lung. Jpn J Clin Oncol. 2011;41:890–6.
Nguyen XC, Lee WW, Chung JH, Park SY, Sung SW, Kim YK, et al. FDG uptake, glucose transporter type 1, and Ki-67 expressions in non-small-cell lung cancer: correlations and prognostic values. Eur J Radiol. 2007;62:214–9.
Uchida T, Rossignol F, Matthay MA, Mounier R, Couette S, Clottes E, et al. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1α and HIF-2α expression in lung epithelial cells: implication of natural antisense HIF-1α. J Biol Chem. 2004;279:14871–8.
Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, et al. Regulation of hypoxia-inducible factor-1α protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3β pathway in HepG2 cells. J Biol Chem. 2003;278:31277–85.
Holmquist-Mengelbier L, Fredlund E, Löfstedt T, Noguera R, Navarro S, Nilsson H, et al. Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell. 2006;10:413–23.
Kim WY, Perera S, Zhou B, Carretero J, Yeh JJ, Heathcote SA, et al. HIF2α cooperates with RAS to promote lung tumorigenesis in mice. J Clin Invest. 2009;119:2160–70.
Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31.
Kamlah F, Eul BG, Li S, Lang N, Marsh LM, Seeger W, et al. Intravenous injection of siRNA directed against hypoxia-inducible factors prolongs survival in a Lewis lung carcinoma cancer model. Cancer Gene Ther. 2009;16:195–205.
Acknowledgments
This study was partially supported by a grant-in-aid for scientific research (26460442) to YU from the Ministry of Education, Science, and Culture, Japan and a grant for specially promoted research from Kanazawa Medical University (SR2012-01) to YU.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Higashi, K., Yamagishi, T., Ueda, Y. et al. Correlation of HIF-1α/HIF-2α expression with FDG uptake in lung adenocarcinoma. Ann Nucl Med 30, 708–715 (2016). https://doi.org/10.1007/s12149-016-1116-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-016-1116-5