Abstract
The co-inhibitory immune checkpoint interaction between programmed cell death-protein 1 (PD-1) and programmed cell death-ligand 1 (PD-L1) serves to regulate T-cell activation, promoting self-tolerance. Over-expression of PD-L1 is a mechanism through which tumour cells can evade detection by the immune system. Several therapeutic antibodies targeting PD-L1 or PD-1 have been approved for the treatment of a variety of cancers, however, the discovery and development of small-molecule inhibitors of PD-L1 remains a challenge. Here we report comprehensive sequence-specific backbone resonance assignments (1H, 13C, and 15N) obtained for the N-terminal IgV-like domain of PD-L1 (D1) and the full two domain extracellular region (D1D2). These NMR assignments will serve as a useful tool in the discovery of small-molecule therapeutics targeting PD-L1 and in the characterisation of functional interactions with other protein partners, such as CD80.
Similar content being viewed by others
Introduction
Human PD-L1 is a 272 amino acid, single-pass transmembrane protein, with two N-terminal Ig-like domains forming the extracellular region (residues 19–239). PD-L1 is the ligand for PD-1 (265 residues), which is also a single-pass transmembrane protein, with a single Ig-like domain forming the extracellular region (amino acids 24–170). The interaction between PD-1 and PD-L1 has been shown to be facilitated by the most N-terminal IgV-like domain of PD-L1 (D1) and the extracellular Ig-like domain of PD-1 (Lin et al. 2008), which regulates a key immune checkpoint, promoting self-tolerance and protecting from auto-immune responses. These effects are achieved by modulating the threshold of T-cell activation via intracellular signalling through PD-1 expressed on T-cells, which is inhibitory to T-cell Receptor signalling (Okazaki and Honjo 2007).
Over-expression of human PD-L1 by tumour cells has been shown to be a key mechanism by which cancers can evade detection by the immune system (Freeman et al. 2000) and has been seen on the surface of many different tumour types, including melanoma, non-small cell lung cancer and lymphoma (Konishi et al. 2004; Nakanishi et al. 2007). Targeting immune checkpoint regulators such as PD-1 and PD-L1 with monoclonal antibodies has revolutionised the treatment of a number of cancers (Topalian et al. 2012; Brahmer et al. 2012; Robert et al. 2015). To date, seven antibodies selected for potent inhibition of PD-1 binding to PD-L1 have been approved as therapeutics, with three targeting the extracellular region of PD-L1 (Upadhaya et al. 2022). Despite highly beneficial clinical responses there are several drawbacks associated with the therapeutic antibodies, including adverse auto-immune effects due to long half-lives in vivo (Naidoo et al. 2015) and problems with tumour penetration (Tan et al. 2016). The discovery and development of specific small-molecule inhibitors of the interaction of PD-L1 with PD-1 has the potential to overcome these problems but remains a major challenge.
PD-L1 has also been found to bind to the extracellular region of membrane-bound CD80 (residues 35–242), which appears to be limited in vivo to when both proteins are on the surface of the same cell (Chaudri et al. 2018). The interaction of PD-L1 with CD80 has been shown to prevent PD-L1 binding to PD-1 and is therefore stimulatory to T-cell responses (Sigiura et al. 2019). The comprehensive backbone NMR assignments reported for the extracellular region of PD-L1 here are expected to be useful tools for both small molecule drug discovery and for further characterisation of interactions with functional partner proteins such as CD80.
Methods and experiments
Protein expression and purification
The coding regions for human PD-L1 (D1: 19–134) and PD-L1 (D1D2: 19–239) were synthesised and cloned into pET28a by GenScript, with codon usage optimised for expression in Escherichia coli. For triple resonance NMR experiments, uniformly 15N/13C-labelled PD-L1 (D1) and 2H/15N/13C-labelled PD-L1 (D1D2) were expressed as insoluble inclusion bodies in appropriately transformed E coli BL21 (DE3) cells grown in M9 minimal medium containing 3 g/l 13C glucose and 1 g/l 15N ammonium sulphate. Deuterated samples were prepared from cells grown in minimal media prepared using > 99% D2O. The BL21 (DE3) cells were cultured at 37° C and protein expression was induced with 1 mM IPTG at an optical density of 0.8–1.0 at 600 nm. The cells were then cultured for a further 5 hours before harvesting by centrifugation. For refolding and purification of PD-L1, cell pellets were resuspended in PBS at pH 7.2 and lysed by sonication before the inclusion bodies were collected by centrifugation. Inclusion bodies were washed twice with 50 mM tris-HCl, 200 mM NaCl, 0.5% triton-X100, 10 mM EDTA and 10 mM DTT at pH 8.0 and once in the same buffer without triton-X100. Washed inclusion bodies were then resolubilised in 50 mM tris-HCl, 5 M guanidine-HCl, 200 mM NaCl and 20 mM DTT at pH 8.0 prior to refolding by drop-wise 100-fold dilution into 0.1 M tris-HCl, 1 M arginine, 0.25 mM oxidised glutathione, and 0.25 mM reduced glutathione at pH 8.0 for PD-L1 (D1) (Zak et al. 2015) and for PD-L1 (D1D2) into the same buffer but with 0.5 mM oxidised glutathione and 2 mM reduced glutathione. After slow stirring for 18 hours at 4° C, refolding mixtures were concentrated by tangential flow filtration, dialysed into a 25 mM potassium phosphate, 20 mM sodium chloride, 10 μM EDTA and 0.02% sodium azide (w/v) buffer at pH 7.5 prior to final purification by size exclusion chromatography on a Superdex-75 column (GE Healthcare).
NMR spectroscopy
3D NMR experiments were acquired from 190 μM and 240 μM samples of uniformly 15N/13C labelled PD-L1 (D1) and 2H/15N/13C labelled PD-L1 (D1D2) respectively, in a 25 mM potassium phosphate, 20 mM sodium chloride, 10 μM EDTA and 0.02% sodium azide (w/v) buffer at pH 7.5, containing 5% D2O. All NMR experiments for PD-L1 (D1) were collected at 298 K whereas NMR experiments for PD-L1 (D1D2) were recorded at 303 K. Sequential backbone resonance assignments for PD-L1 (D1) were determined using a combination of 15N/13C/1H HNCACB (Wittekind and Mueller 1993), CBCA(CO)NH (Grezesiek and Bax 1992) and HNCO (Kay et al. 1990) spectra. Assignments for PD-L1 (D1D2) were determined using a combination of 15N/13C/1H TROSY-HNCACB, TROSY-HN(CO)CACB (Yamazaki et al. 1994) and TROSY-HNCO spectra. Linear prediction was used to extend the effective acquisition time in 15N by two fold in the CBCA(CO)NH and HNCO experiments for PD-L1 (D1) and in the TROSY-HNCO for PD-L1 (D1D2). The acquisition parameters used for all NMR experiments are summarised in Table 1. NMR experiments were processed using NMRPipe (Delaglio et al. 1995). Where non-uniform sampling (NUS) was used during data collection, the data was reconstructed using NMRPipe’s Interative Shrinkage Thresholding (IST) software. All analysis of spectra was carried out manually using NMRFAM-SPARKY (Lee et al. 2015).
Extent of assignments and data deposition
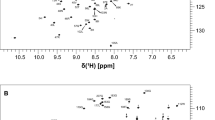
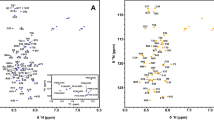
Comprehensive sequence-specific backbone resonance assignments (90% HN, 90% N, 90% C’, 92% Cα and 92% Cβ) were obtained for PD-L1 (D1) with backbone amide assignments made for 101 of the 112 non-proline residues (Fig. 1). For the majority of the residues in PD-L1 (D1) with unassigned backbone amide signals this is due to associated peaks being absent from the NMR spectra acquired due to conformational dynamics, resulting in substantial broadening of amide resonances. For example, backbone amide signals could not be assigned for residues C40 to K46, which form part of a long solvent accessible loop connecting β-strands B and C in PD-L1 (D1) (Fig. 2) with the potential for significant conformational heterogeneity. The sequence-specific backbone NMR assignments obtained for PD-L1 (D1) have been deposited in the BioMagResBank (http://www.bmrb.wisc.edu) under accession number 51412.
A typical 15N/1H HSQC spectrum of PD-L1 (D1) (190 µM) in a 25 mM potassium phosphate, 20 mM sodium chloride, 10 μM EDTA and 0.02% sodium azide (w/v) buffer at pH 7.5 containing 5% D2O. The spectrum was acquired at 298 K and 800 MHz. The sequence-specific assignments obtained for backbone amide signals are indicated in the contour plot
Somewhat less complete sequence specific backbone assignments were obtained for PD-L1 (D1D2) (79% HN, 79% N, 75% C’, 80% Cα and 79% Cβ), with backbone amide assignments made for 167 of the 211 non-proline residues (Fig. 3). This is primarily due to assignment of only 75% of the backbone amide resonances expected from the membrane proximal domain of PD-L1 (D2). The non-assigned signals are predominantly associated with residues forming the loops connecting β-strands B and C, D and E, and β-strand D (Fig. 4). Given the absence of these signals in the 3D NMR spectra, it seems likely that this region of PD-L1 (D2) is exchanging between multiple conformational states and/or exchanging with the solvent, resulting in broadening of NMR signals beyond detection. The sequence-specific backbone NMR assignments obtained for PD-L1 (D1D2) have been deposited in the BioMagResBank under accession number 51411.
A typical 15N/1H TROSY spectrum of PD-L1 (D1D2) (240 µM) in a 25 mM potassium phosphate, 20 mM sodium chloride, 10 μM EDTA and 0.02% sodium azide (w/v) buffer at pH 7.5 containing 5% D2O. The spectrum was acquired at 303 K and 950 MHz. The sequence-specific assignments obtained for backbone amide signals are indicated in the contour plot
TALOS-N was used to predict the secondary structure of both PD-L1 (D1) and PD-L1 (D1D2) using primarily the NMR assignments obtained (Shen and Bax 2013), however, predictions for non-assigned residues were based on the protein sequence (Fig. 5). For PD-L1 (D1D2), the NMR-based predictions show good agreement with the crystal structure reported for PD-L1 (D1D2) (Fig. 6), PDB: 3FN3, (Chen et al. 2010). TALOS-N analysis of the NMR data predicts an additional α-helix from K185-E188, however, for these residues this is based on sequence alone. In the case of PD-L1 (D1), the secondary structure predicted by analysis of the NMR data also showed good agreement with the crystal structure reported for PD-L1 (D1) (Fig. 6) PDB: 5C3T (Zak et al. 2015).
Predicted secondary structure of PD-L1_D1 (A) and PD-L1_D1D2 (B) based on the analysis of sequence-specific backbone NMR assignments (N, NH, CO, Cα, and Cβ) obtained for both proteins using TALOS-N. Predicted ⍺-helical structure is shown as positive values (red) and predicted β-strands as negative values (blue). For a limited number of residues, the predicted secondary structure is based on the protein sequence alone, which is indicated by paler red or blue bars
It is our hope that these backbone NMR assignments will be a useful tool for the study of the interactions of PD-L1 with functional partners as well as in the development of small-molecule therapeutics targeting PD-L1.
Data availability
The sequence-specific backbone NMR assignments obtained for PD-L1 (D1) have been deposited in the BioMagResBank (http://www.bmrb.wisc.edu) under accession number 51412. The sequence-specific backbone NMR assignments obtained for PD-L1 (D1D2) have been deposited in the BioMagResBank under accession number 51411.
References
Brahmer JR, Tykodi SS, Chow LQM, Hwu WJ, Topalian S, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC et al (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Eng J Med 366(26):2455–2465. https://doi.org/10.1056/NEJMoa1200694
Chaudri A, Xiao Y, Klee AN, Wang X, Zhu B, Freeman GJ (2018) PD-L1 Binds to B7–1 Only in cis on the same cell surface. Cancer Immunol Res 6(8):921–929. https://doi.org/10.1158/2326-6066.CIR-17-0316
Chen Y, Liu P, Gao F, Cheng H, Qi J, Gao GF (2010) A dimeric structure of PD-L1: functional units or evolutionary relics? Protein Cell 1(2):153–160. https://doi.org/10.1007/s13238-010-0022-1
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6(3):277–93. https://doi.org/10.1007/BF00197809
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T (2000) Engagement of the Pd-1 immunoinhibitory receptor by a novel b7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192(7):1072–1034. https://doi.org/10.1084/jem.192.7.1027
Grezesiek S, Bax A (1992) Correlating backbone amide and side chain resonances in larger proteins by multiple relayed triple resonance NMR. J Am Chem Soc 114(16):6291–6293. https://doi.org/10.1021/ja00042a003
Kay LE, Ikura M, Tschudin R, Bax A (1990) Three-dimensional triple-resonance NMR spectroscopy of isotopically enriched proteins. J Magn Reson 89(3):496–514. https://doi.org/10.1016/0022-2364(90)90333-5
Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Masaharu Nishimura (2004) B7–H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 10(15):5094–5100. https://doi.org/10.1158/1078-0432.CCR-04-0428
Lee W, Tonelli M, Markley JL (2015) NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics 31(8):1325–1327. https://doi.org/10.1093/bioinformatics/btu830
Lin YWD, Tanaka Y, Iwasaki M, Gittis AG, Su HP, Mikami B, Okazaki T, Honjo T, Minato N, Garboczi DN (2008) The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. PNAS 105(8):3011–3016. https://doi.org/10.1073/pnas.0712278105
Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD (2015) Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26(12):2375–2391. https://doi.org/10.1093/annonc/mdv383
Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S (2007) Overexpression of B7–H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother 56(8):1173–1182. https://doi.org/10.1007/s00262-006-0266-z
Okazaki T, Honjo T (2007) PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 19(7):813–824. https://doi.org/10.1093/intimm/dxm057
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM et al (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Eng J Med 372:320–330. https://doi.org/10.1056/NEJMoa1412082
Shen Y, Bax A (2013) Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J Biomol NMR 56:227–241. https://doi.org/10.1007/s10858-013-9741-y
Sigiura D, Maruhashi T, Okazaki IM, Shimizu K, Maeda TK, Takemoto T, Okazaki T (2019) Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science 364(6440):558–566. https://doi.org/10.1126/science.aav7062
Tan S, Zhang CWH, Gao GF (2016) Seeing is believing: anti-PD-1/PD-L1 monoclonal antibodies in action for checkpoint blockade tumor immunotherapy. Sig Transduct Target Ther 1:16029. https://doi.org/10.1038/sigtrans.2016.29
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Eng J Med 366(26):2443–2454. https://doi.org/10.1056/NEJMoa1200690
Upadhaya S, Neftelinov ST, Hodge J, Campbell J (2022) Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat Rev Drug Discov. https://doi.org/10.1038/d41573-022-00030-4
Wittekind M, Mueller L (1993) HNCACB, a high-sensitivity 3D NMR experiment to correlate amide-proton and nitrogen resonances with the alpha- and beta-carbon resonances in proteins. J Magn Res 101(2):201–205. https://doi.org/10.1006/jmrb.1993.1033
Yamazaki T, Lee W, Arrowsmith CH, Muhandiram DR, Kay LE (1994) A suite of triple resonance nmr experiments for the backbone assignment of 15N, 13C, 2H labeled proteins with high sensitivity. J Am Chem Soc 116(26):11655–11666. https://doi.org/10.1021/ja00105a005
Zak KM, Kitel R, Przetocka S, Golik P, Guzik K, Musielak B, Dömling A, Dubin G, Holak TA (2015) Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1. Structure 23(12):2341–2348. https://doi.org/10.1016/j.str.2015.09.010
Acknowledgements
The work reported here was undertaken as part of a UK Medical Research Council PhD studentship awarded to Kayleigh Walker. NMR experiments collected at 950 MHz were carried out at the MRC Biomedical NMR Centre based at the Francis Crick Institute in London.
Funding
This work was funded by the UK Medical Research Council.
Author information
Authors and Affiliations
Contributions
KW and MDC contributed to the study conceptualisation and design. Data collection and analysis was performed by KW, LCW, FWM and GK. KW wrote the manuscript, and this was reviewed by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Walker, K., Waters, L.C., Kelly, G. et al. Sequence-specific 1H, 13C and 15N backbone NMR assignments for the N-terminal IgV-like domain (D1) and full extracellular region (D1D2) of PD-L1. Biomol NMR Assign 16, 281–288 (2022). https://doi.org/10.1007/s12104-022-10092-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-022-10092-5