Abstract
Background
Brain tumors represent the most common cause of cancer-related death in children. Few studies concerning the palliative phase in children with brain tumors are available.
Objectives
(i) To describe the palliative phase in children with brain tumors; (ii) to determine whether the use of palliative sedation (PS) depends on the place of death, the age of the patient, or if they received specific palliative care (PC).
Methods
Retrospective multicenter study between 2010 and 2021, including children from one month to 18 years, who had died of a brain tumor.
Results
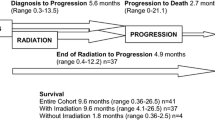
228 patients (59.2% male) from 10 Spanish institutions were included. Median age at diagnosis was 5 years (IQR 2–9) and median age at death was 7 years (IQR 4–11). The most frequent tumors were medulloblastoma (25.4%) and diffuse intrinsic pontine glioma (DIPG) (24.1%). Median number of antineoplastic regimens were 2 (range 0–5 regimens). During palliative phase, 52.2% of the patients were attended by PC teams, while 47.8% were cared exclusively by pediatric oncology teams. Most common concerns included motor deficit (93.4%) and asthenia (87.5%) and communication disorders (89.8%). Most frequently prescribed supportive drugs were antiemetics (83.6%), opioids (81.6%), and dexamethasone (78.5%). PS was administered to 48.7% patients. Most of them died in the hospital (85.6%), while patients who died at home required PS less frequently (14.4%) (p = .01).
Conclusion
Children dying from CNS tumors have specific needs during palliative phase. The optimal indication of PS depended on the center experience although, in our series, it was also influenced by the place of death.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, [L.M], upon reasonable request.
Abbreviations
- CNS:
-
Central nervous system
- PS:
-
Palliative sedation
- PC:
-
Palliative care
- SEHOP:
-
Spanish Society of Pediatric Onco-Hematology
- IQR:
-
Interquartile range
- DIPG:
-
Diffuse intrinsic pontine glioma
- VEGF:
-
Vascular endothelial growth factor
- ITCC:
-
Innovative therapies for children with cancer
- VPS:
-
Ventriculo-peritoneal shunting
- NICE:
-
National Institute for Health and Care Excellence
References
Peris-Bonet R, Martínez-García C, Lacour B, Petrovich S, Giner-Ripoll B, Navajas A, et al. Childhood central nervous system tumours—incidence and survival in Europe (1978–1997): report from Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):2064–80.
Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer CA Cancer J Clin. 2014;64(2):83–103.
Groh G, Feddersen B, Führer M, Borasio GD. Specialized home palliative care for adults and children: differences and similarities. J Palliat Med. 2014;17(7):803–10.
Vallero SG, Lijoi S, Bertin D, Pittana LS, Bellini S, Rossi F, et al. End-of-life care in pediatric neuro-oncology. Pediatr Blood Cancer. 2014;61(11):2004–11.
Zelcer S, Cataudella D, Cairney AE, Bannister SL. Palliative care of children with brain tumors: a parental perspective. Arch Pediatr Adolesc Med. 2010;164(3):225–30.
Kuhlen M, Hoell J, Balzer S, Borkhardt A, Janssen G. Symptoms and management of pediatric patients with incurable brain tumors in palliative home care. Eur J Paediatr Neurol. 2016;20(2):261–9.
Jagt-van Kampen CT, van de Wetering MD, Schouten-van Meeteren AYN. The timing, duration, and management of symptoms of children with an incurable brain tumor: a retrospective study of the palliative phase. Neuro Oncol. 2015;2(2):70–7.
de Noriega I, Martino Alba R, Herrero Velasco B, Madero López L, Lassaletta Á. Palliative care in pediatric patients with central nervous system cancer: descriptive and comparative study. Palliat Support Care. 2022;12:1–8.
OrtizSanRomán L, Martino ARJ. Enfoque paliativo en Pediatría. Pediatr Integral. 2016;XX(2):131–7.
World Health Organization. Integrating palliative care and symptom relief into paediatrics: a WHO guide for health-care planners, implementers and managers. World Health Organization. (2018). https://apps.who.int/iris/handle/10665/274561. License: CC BY-NC-SA 3.0 IGO. Accessed 10 Oct 2020.
Gómez Sancho M, Altisent Trota R, Bátiz Cantera J, Ciprés-Casasnovas L, Gándara-del-Castillo A, Herranz-Martínez JA, et al. “Atención Médica al final de la vida: conceptos y definiciones”. Grupo de trabajo “Atención médica al final de la vida”. Organización Médica Colegial (OMC) y Sociedad Española de Cuidados Paliativos (SECPAL)Gac Med Bilbao. 2015;112(4):216–18.
Rodríguez Hernández PJ, Barrau Alonso VM. Trastornos del comportamiento. Pediatr Integral. 2012;XVI(10):760–8.
Martínez MN. Trastornos depresivos en niños y adolescentes. An Pediatr Contin. 2014;12(6):294–9.
Bernaras E, Jaureguizar J, Garaigordobil M. Child and adolescent depression: a review of theories, evaluation instruments, prevention programs, and treatments. Front Psychol. 2019;10(543):1–24.
Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(2):160–74.
[Internet]. World Health Organization; [Cited 9th of july 2021]. https://www.who.int/es/news-room/fact-sheets/detail/malnutrition. Accessed 5 Nov 2020.
Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–5.
Gore M, Dukes E, Rowbotham DJ, Tai KS, Leslie D. Clinical characteristics and pain management among patients with painful peripheral neuropathic disorders in general practice settings. Eur J Pain. 2007;11(6):652–64.
Dees MK, Vernooij-Dassen MJ, Dekkers WJ, Vissers KC, van Weel C. ‘Unbearable suffering’: a qualitative study on the perspectives of patients who request assistance in dying. J Med Ethics. 2011;37(12):727–34.
Voeuk A, Nekolaichuk C, Fainsinger R, Huot A. Continuous palliative sedation for existential distress? A survey of Canadian palliative care physicians’ views. J Palliat Care. 2017;32(1):26–33.
Hongo T, Watanabe C, Okada S, Inoue N, Yajima S, Fujii Y, Ohzeki T. Analysis of the circumstances at the end of life in children with cancer: symptoms, suffering and acceptance. Pediatr Int. 2003;45(1):60–4.
Goldman A, Hewitt M, Collins GS, Childs M, Hain R. Symptoms in children/young people with progressive malignant disease: United Kingdom Children’s Cancer Study Group/Paediatric Oncology Nurses Forum survey. Pediatrics. 2006;117(6):1179–86.
Portela Tejedor MA, Sanz Rubiales A, Martínez M, Centeno C. Astenia en cáncer avanzado y uso de psicoestimulantes. An Sist Sanit Navar. 2011;34(3):471–9.
Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30:48–57.
Beretta S, Polastri D, Clerici CA, Casanova M, Cefalo G, Ferrari A, et al. End of life in children with cancer: experience at the pediatric oncology department of the istituto nazionale tumori in Milan. Pediatr Blood Cancer. 2010;54(1):88–91.
Jalmsell L, Kreicbergs U, Onelöv E, Steineck G, Henter JI. Symptoms affecting children with malignancies during the last month of life: a nationwide follow-up. Pediatrics. 2006;117(4):1314–20.
Jessurun CAC, Hulsbergen AFC, Cho LD, Aglio LS, Nandoe Tewarie RDS, Broekman MLD. Evidence-based dexamethasone dosing in malignant brain tumors: what do we really know? J Neurooncol. 2019;144(2):249–64.
Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4(2):233–42.
Delishaj D, Ursino S, Pasqualetti F. Bevacizumab for the treatment of radiation-induced cerebral necrosis: a systematic review of the literature. J Clin Med Res. 2017;9(4):273–80.
Baroni LV, Alderete D, Solano-Paez P, Rugilo C, Freytes C, Laughlin S, et al. Bevacizumab for pediatric radiation necrosis. Neurooncol Pract. 2020;7(4):409–14.
Barone A, Rubin JB. Opportunities and challenges for successful use of bevacizumab in pediatrics. Front Oncol. 2013;3:92.
Carceller F, Fowkes LA, Khabra K, Moreno L, Saran F, Burford A, et al. Pseudoprogression in children, adolescents and young adults with non-brainstem high grade glioma and diffuse intrinsic pontine glioma. J Neurooncol. 2016;129(1):109–21.
Foster KA, Ares WJ, Pollack IF, Jakacki RI. Bevacizumab for symptomatic radiation-induced tumor enlargement in pediatric low grade gliomas. Pediatr Blood Cancer. 2015;62(2):240–5.
Morgan KJ, Anghelescu DL. A review of adult and pediatric neuropathic pain assessment tools. Clin J Pain. 2017;33(9):844–52.
Walker SM. Neuropathic pain in children: steps towards improved recognition and management. EBioMedicine. 2020;62: 103124.
Moreno L, Pearson ADJ, Paoletti X, Jimenez I, Geoerger B, Kearns PR, et al. Innovative Therapies for Children with Cancer (ITCC) Consortium. Early phase clinical trials of anticancer agents in children and adolescents—an ITCC perspective. Nat Rev Clin Oncol. 2017;14(8):497–507.
Quiroga Cantero E, Moreno Retortillo L, Martino AR. Ensayos clínicos y cuidados paliativos pediátricos. Med Paliat. 2019;26(2):95–6.
Benedetti DJ, Marron JM. Ethical challenges in pediatric oncology care and clinical trials. Recent Results Cancer Res. 2021;218:149–73.
Bautista F, Gallego S, Cañete A, Mora J, Díaz de Heredia C, Cruz O, et al, en nombre de la Sociedad Española de Oncología Pediátrica (SEHOP) y el Grupo de Nuevas Terapias en Oncología Pediátrica; Sociedad Española de Hematología y Oncología Pediátrica (SEHOP) and the New Drug Development Group in Pediatric Oncology. Ensayos clínicos precoces en oncología pediátrica en España: una perspectiva nacional [Early clinical trials in paediatric oncology in Spain: a nationwide perspective]. An Pediatr (Barc). 2017;87(3):155–63 (Spanish).
Levine DR, Johnson LM, Mandrell BN, Yang J, West NK, Hinds PS, Baker JN. Does phase 1 trial enrollment preclude quality end-of-life care? Phase 1 trial enrollment and end-of-life care characteristics in children with cancer. Cancer. 2015;121(9):1508–12.
Kaye EC, Jerkins J, Gushue CA, DeMarsh S, Sykes A, Lu Z, et al. Predictors of late palliative care referral in children with cancer. J Pain Symptom Manag. 2018;55(6):1550–6.
Schiff D, Kline C, Meltzer H, Auger J. Palliative ventriculoperitoneal shunt in a pediatric patient with recurrent metastatic medulloblastoma. J Palliat Med. 2009;12(4):391–3.
Fonseca A, Solano P, Ramaswamy V, Tabori U, Huang A, Drake JM, et al. Ventricular size determination and management of ventriculomegaly and hydrocephalus in patients with diffuse intrinsic pontine glioma: an institutional experience. J Neurosurg. 2021;135(4):1139–45.
Kim HS, Park JB, Gwak HS, Kwon JW, Shin SH, Yoo H. Clinical outcome of cerebrospinal fluid shunts in patients with leptomeningeal carcinomatosis. World J Surg Oncol. 2019;17(1):59.
Bluebond-Langner M, Beecham E, Candy B, Langner R, Jones L. Preferred place of death for children and young people with life-limiting and life-threatening conditions: a systematic review of the literature and recommendations for future inquiry and policy. Palliat Med. 2013;27(8):705–13.
Levine DR, Mandrell BN, Sykes A, Pritchard M, Gibson D, Symons HJ, et al. Patients’ and parents’ needs, attitudes, and perceptions about early palliative care integration in pediatric oncology. JAMA Oncol. 2017;3(9):1214–20.
Navarro-Vilarrubí S. Desarrollo de la atención paliativa, imparable en pediatría. An Pediatr (Barc). 2022;96(5):383–4.
Peláez Cantero MJ, Morales Asencio JM, NavarroMarchena L, del Velázquez González MR, Sánchez Echàniz J, Rubio Ortega L, et al. El final de vida en pacientes atendidos por equipos de cuidados paliativos pediátricos. Estudio observacional multicéntrico. An Pediatr (Barc). 2022;96(5):383–410.
Villanueva G, Murphy MS, Vickers D, Harrop E, Dworzynski K. End of life care for infants, children and young people with life limiting conditions: summary of NICE guidance. BMJ. 2016;355: i6385.
Pousset G, Bilsen J, Cohen J, Mortier F, Deliens L. Continuous deep sedation at the end of life of children in Flanders, Belgium. J Pain Symptom Manag. 2011;41(2):449–55.
Kiman R, Wuiloud AC, Requena ML. End of life care sedation for children. Curr Opin Support Palliat Care. 2011;5(3):285–90.
Korzeniewska-Eksterowicz A, Przysło Ł, Fendler W, Stolarska M, Młynarski W. Palliative sedation at home for terminally ill children with cancer. J Pain Symptom Manag. 2014;48(5):968–74.
Postovsky S, Moaed B, Krivoy E, Ofir R, Ben Arush MW. Practice of palliative sedation in children with brain tumors and sarcomas at the end of life. Pediatr Hematol Oncol. 2007;24(6):409–15.
Cuviello A, Johnson LM, Morgan KJ, Anghelescu DL, Baker JN. Palliative sedation therapy in pediatrics: an algorithm and clinical practice update. Children (Basel). 2022;9(12):1887.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by MP-TL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethics approval
This retrospective chart review study involving human participants was performed in line with the principles of the Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not required since the study is retrospective, it doesn’t use identifiable private information or identifiable biospecimens and the research doesn’t involves any risk to the subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pérez-Torres Lobato, M., Navarro-Marchena, L., de Noriega, I. et al. Palliative care for children with central nervous system tumors: results of a Spanish multicenter study. Clin Transl Oncol 26, 786–795 (2024). https://doi.org/10.1007/s12094-023-03301-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03301-7