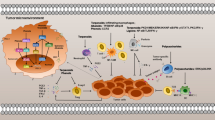
Abstract
In modern medicine, natural products have aided humans against their battles with cancer. Among these products, microorganisms, medicinal herbs and marine organisms are considered to be of great benefit. In recent decades, more than 30 fungal immunity proteins have been identified and proved to be extractable from a wide range of fungi, including mushrooms. Although chemotherapy is used to overcome cancer cells, the side effects of this method are of great concern in clinical practice. Fungal products and their derivatives constitute more than 50% of the clinical drugs currently being used globally. Approximately 60% of the clinically approved drugs for cancer treatment have natural roots. Anti-tumor immunotherapy is prospective with a rapidly growing market worldwide due to its high efficiency, immunity, and profit. Polysaccharide extracts from natural sources are being used in clinical and therapeutic trials on cancer patients. This review aims to present the latest findings in cancer treatment through isolated and extraction of fungal derivatives and other natural biomaterials.


Similar content being viewed by others
References
Vaca I, Chávez R. Bioactive compounds produced by Antarctic filamentous fungi. In: Rosa LH, editor. Fungi of Antarctica. Cham: Springer; 2019. p. 265–83.
Salehi B, Bayat M, Dezfulian M, Sabokbar A, Tabaraie B. The assessment of anti-tumoral activity of polysaccharide extracted from terrestrial filamentous fungus. Saudi J Biol Sci. 2018;25(6):1236–41.
Stanojković T. Investigations of lichen secondary metabolites with potential anticancer activity. In: Ranković B, editor. Lichen secondary metabolites. Cham: Springer; 2019. p. 155–74.
Saravanakumar K, Shanmugam S, Varukattu NB, MubarakAli D, Kathiresan K, Wang M-H. Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J Photochem Photobiol B. 2019;190:103–9.
Chen L, Zhang Q-Y, Jia M, Ming Q-L, Yue W, Rahman K, et al. Endophytic fungi with antitumor activities: their occurrence and anticancer compounds. Crit Rev Microbiol. 2016;42(3):454–73.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2018;68(6):394–424.
Yan J-K, Pei J-J, Ma H-L, Wang Z-B, Liu Y-S. Advances in antitumor polysaccharides from Phellinus sensu lato: production, isolation, structure, antitumor activity, and mechanisms. Crit Rev Food Sci Nutr. 2017;57(6):1256–69.
Masuda Y, Nakayama Y, Tanaka A, Naito K, Konishi M. Antitumor activity of orally administered maitake α-glucan by stimulating antitumor immune response in murine tumor. PLoS ONE. 2017;12(3):e0173621.
Li Q-Z, Zheng Y-Z, Zhou X-W. Fungal immunomodulatory proteins: characteristic, potential antitumor activities and their molecular mechanisms. Drug Discov Today. 2019;24(1):307–14.
Matuszewska A, Stefaniuk D, Jaszek M, Pięt M, Zając A, Matuszewski Ł, et al. Antitumor potential of new low molecular weight antioxidative preparations from the white rot fungus Cerrena unicolor against human colon cancer cells. Sci Rep. 2019;9(1):1–10.
Ahmad MF. Ganoderma lucidum: a macro fungus with phytochemicals and their pharmacological properties. In: Ozturk M, Hakeem KR, editors. Plant and human health. vol. 2. Cham: Springer; 2019. pp. 491–515
Rajamanikyam M, Vadlapudi V, Upadhyayula SM. Endophytic fungi as novel resources of natural therapeutics. Braz Arch Biol Technol. 2017;60:e17160542.
Madhanraj R, Ravikumar K, Maya M, Illuri R, Venkatakrishna K, Rameshkumar K, et al. Evaluation of anti-microbial and anti-haemolytic activity of edible basidiomycetes mushroom fungi. J Drug Deliv Ther. 2019;9(1):132–5.
Li W, Song K, Wang S, Zhang C, Zhuang M, Wang Y, et al. Anti-tumor potential of astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater Sci Eng C. 2019;98:685–95.
Maehara S, Yamane C, Kitamura C, Hinokuma M, Hata T. High ophiobolin A production in endophytic fungus Bipolaris sp. associated with Datura metel. Nat Prod Res. 2019;33:1–3.
Noh S, Choi E, Hwang C-H, Jung JH, Kim S-H, Kim B. Dietary compounds for targeting prostate cancer. Nutrients. 2019;11(10):2401.
Matuszewska A, Stefaniuk D, Jaszek M, Pięt M, Zając A, Matuszewski Ł, et al. Antitumor potential of new low molecular weight antioxidative preparations from the white rot fungus Cerrena unicolor against human colon cancer cells. Sci Rep. 2019;9:1975.
Vil VA, Terent’ev AO, Savidov N, Gloriozova TA, Poroikov VV, Pounina TA, et al. Hydroperoxy steroids and triterpenoids derived from plant and fungi: origin, structures and biological activities. J Steroid Biochem Mol Biol. 2019;190:76–87.
Wang C, Zhang W, Wong JH, Ng T, Ye X. Diversity of potentially exploitable pharmacological activities of the highly prized edible medicinal fungus Antrodia camphorata. Appl Microbiol Biotechnol. 2019;103(19):7843–67.
Yang X-Y, Niu W-R, Li R-T, Cui X-M, Liu J-K. Two new sesquiterpenes from cultures of the higher fungus Pholiota nameko. Nat Prod Res. 2019;33(14):1992–6.
Vannucci L, Krizan J, Sima P, Stakheev D, Caja F, Rajsiglova L, et al. Immunostimulatory properties and antitumor activities of glucans. Int J Oncol. 2013;43(2):357–64.
Janakiraman V, Govindarajan K, Magesh C. Biosynthesis of silver nanoparticles from endophytic fungi, and its cytotoxic activity. BioNanoScience. 2019;9(3):573–9.
Mohseni MS, Khalilzadeh MA, Mohseni M, Hargalani FZ, Getso MI, Raissi V, et al. Green synthesis of Ag nanoparticles from pomegranate seeds extract and synthesis of Ag-Starch nanocomposite and characterization of mechanical properties of the films. Biocatal Agric Biotechnol. 2020;25:101569.
Barabadi H, Mahjoub MA, Tajani B, Ahmadi A, Junejo Y, Saravanan M. Emerging theranostic biogenic silver nanomaterials for breast cancer: a systematic review. J Clust Sci. 2019;30(2):259–79.
El-Kahky D, Attia M, Easa SM, Awad NM, Helmy EA. Biosynthesized of zinc oxide nanoparticles using Aspergillus terreus and their application as antitumor and antimicrobial activity. J Ecol Eng. 2019;8(3):90–100.
Peng C, Han B, Wang B, Zhao G, Yuan F, Zhao Y, et al. Therapy of prostate cancer by nanoyam polysaccharide. Int J Polym Sci. 2019;2019:1–5.
Husseiny SM, Salah TA, Anter HA. Biosynthesis of size controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. Beni-Suef Univ J Basic Appl Sci. 2015;4(3):225–31.
Benchamin D, Sreejai R, Sujitha S, Jensy Roshan F, Albert C, Rishad K. Anti-proliferative activity of L-Asparaginase enzyme from fungi on breast cancer. J Pharmacogn Phytochem. 2019;8(1):407–10.
Erden Y, Tekin S, Ceylan KBB, Tekin C, Kirbag S. Antioxidant, antimicrobial and anticancer activities of the aspergillin PZ and terphenyllin secondary metabolites: an in vitro study. Gazi Univ J Sci. 2019;32(3):792–800.
Meng Q, Zhou X, Ran X, Yan S, Fu S. Microbial synthesis of anti-tumor agent oxysophoridine through one step by filamentous fungus. J Pharm Biopharm Res. 2019;1(2):48–52.
Garrido-Rodríguez M, Ortea I, Calzado MA, Muñoz E, García V. SWATH proteomic profiling of prostate cancer cells identifies NUSAP1 as a potential molecular target for Galiellalactone. J Proteom. 2019;193:217–29.
Antipova TV, Zaitsev KV, Oprunenko YF, Zherebker AY, Rystsov GK, Zemskova MY, et al. Austalides V and W, new meroterpenoids from the fungus Aspergillus ustus and their antitumor activities. Bioorg Med Chem Lett. 2019;29(22):126708.
Lee D, Lee W-Y, Jung K, Kwon YS, Kim D, Hwang GS, et al. The inhibitory effect of cordycepin on the proliferation of MCF-7 breast cancer cells, and its mechanism: an investigation using network pharmacology-based analysis. Biomolecules. 2019;9(9):414.
Chien R-C, Yen M-T, Mau J-L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohyd Polym. 2016;138:259–64.
Choromanska A, Kulbacka J, Rembialkowska N, Pilat J, Oledzki R, Harasym J, et al. Anticancer properties of low molecular weight oat beta-glucan—an in vitro study. Int J Biol Macromol. 2015;80:23–8.
Choromanska A, Kulbacka J, Harasym J, Oledzki R, Szewczyk A, Saczko J. High-and low-molecular weight oat beta-glucan reveals antitumor activity in human epithelial lung cancer. Pathol Oncol Res. 2018;24(3):583–92.
El-Kassem LA, Hawas UW, El-Souda S, Ahmed EF, El-Khateeb W, Fayad W. Anti-HCV protease potential of endophytic fungi and cytotoxic activity. Biocatal Agric Biotechnol. 2019;19:101170.
Karaman M, Janjušević L, Jakovljević D, Šibul F, Pejin B. Anti-hydroxyl radical activity, redox potential and anti-AChE activity of Amanita strobiliformis polysaccharide extract. Nat Prod Res. 2019;33(10):1522–6.
Khan T, Date A, Chawda H, Patel K. Polysaccharides as potential anticancer agents—a review of their progress. Carbohydr Polym. 2019;210:412–28.
Li H, Cao K, Cong P, Liu Y, Cui H, Xue C. Structure characterization and antitumor activity of the extracellular polysaccharide from the marine fungus Hansfordia sinuosae. Carbohyd Polym. 2018;190:87–94.
Liu X-C, Zhu Z-Y, Liu Y-L, Sun H-Q. Comparisons of the anti-tumor activity of polysaccharides from fermented mycelia and cultivated fruiting bodies of Cordyceps militaris in vitro. Int J Biol Macromol. 2019;130:307–14.
Qi C, Gao W, Guan D, Wang J, Liu M, Chen C, et al. Butenolides from a marine-derived fungus Aspergillus terreus with antitumor activities against pancreatic ductal adenocarcinoma cells. Bioorg Med Chem. 2018;26(22):5903–10.
Sun X, Zhao C, Pan W, Wang J, Wang W. Carboxylate groups play a major role in antitumor activity of Ganoderma applanatum polysaccharide. Carbohyd Polym. 2015;123:283–7.
Acknowledgements
Sincere gratitude of all professors and students of parasitology and mycology at the School of Public Health at Tehran University of Medical Sciences.
Funding
Funding information is not applicable/no funding was received.
Author information
Authors and Affiliations
Contributions
OR, SSH, and KA conceived the research; MY, VR, HE, SK, BA, and AS were responsible for literature search, abstract and title screening, full-text review, and extraction of data. OR provided coordination and oversight; ASM, MM, and MM drafted the manuscript. OR, RA, and MG critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare in this work.
Ethical approval
Not applicable; no human or animal subjects were directly involved in this research.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shamsaei, S., Getso, M., Ahmadikia, K. et al. Recent findings on the role of fungal products in the treatment of cancer. Clin Transl Oncol 23, 197–204 (2021). https://doi.org/10.1007/s12094-020-02428-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02428-1