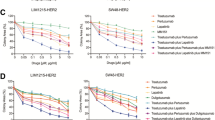
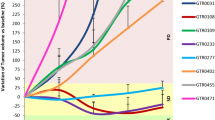
Abstract
Numerous inherent and acquired genetic alterations have been demonstrated with resistance to anti-epidermal growth factor receptor (anti-EGFR) therapy in metastatic colorectal cancer (mCRC) patients. Although the common oncogenic driver mutations identified include KRAS, NRAS, BRAF, and PI3K, recent studies report a vital role played by human epithelial growth factor receptor-2 (HER2) amplification in acquired resistance to anti-EGFR therapy. HER2 amplification has been associated with poor prognosis in many malignancies including breast and gastric cancer and is also a negative predictor of anti-EGFR therapy. Given the relevance of HER2 amplification in conferring an anti-EGFR resistance, this paper reviews the prevalence of HER2 amplification in mCRC while exploring the prognostic and predictive values of this biomarker. Further, we also discuss the results of the studies that explored the utilization of anti-HER2-targeted therapies in mCRC. HER2-directed therapies have the ability to change the treatment algorithm in clinically relevant small subset of patients with HER2-amplified mCRC.

Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Cook AD, Single R, McCahill LE. Surgical Resection of Primary tumors in patients who present with stage iv colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol. 2005;12:637–45.
Misiakos EP, Karidis NP, Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol. 2011;17:4067–75.
Martini G, Troiani T, Cardone C, Vitiello P, Sforza V, Ciardiello D, et al. Present and future of metastatic colorectal cancer treatment: a review of new candidate targets. World J Gastroenterol. 2017;23:4675–88.
Atreya CE, Yaeger R, Chu E. Systemic therapy for metastatic colorectal cancer: from current standards to future molecular targeted approaches. Orlando: American Society of Clinical Oncology Educational Book; 2017. p. 246–256.
Valentini AM, Cavalcanti E, Di Maggio M, Caruso ML. RAS-expanded mutations and HER2 expression in metastatic colorectal cancer: a new step of precision medicine. Appl Immunohistochem Mol Morphol. 2018;26(8):539–44.
Sforza V, Martinelli E, Ciardiello F, Gambardella V, Napolitano S, Martini G, et al. Mechanisms of resistance to anti-epidermal growth factor receptor inhibitors in metastatic colorectal cancer. World J Gastroenterol. 2016;22:6345–61.
Sartore-Bianchi A, Di Nicolantonio F, Nichelatti M, Molinari F, De Dosso S, Saletti P, et al. Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS ONE. 2009;4:e7287.
De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–62.
Douillard J-Y, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III Trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–705.
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S-E, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75.
Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon J-L, Hecht JR, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240–7.
Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A. Resistance to Anti-EGFR Therapy in Colorectal Cancer: From Heterogeneity to Convergent Evolution. Cancer Discov. 2014;4:1269–80.
Hsu H-C, Thiam TK, Lu Y-J, Yeh CY, Tsai W-S, You JF, et al. Mutations of KRAS/NRAS/BRAF predict cetuximab resistance in metastatic colorectal cancer patients. Oncotarget. 2016;7:22257–70.
Rankin A, Klempner SJ, Erlich R, Sun JX, Grothey A, Fakih M, et al. Broad detection of alterations predicted to confer lack of benefit from EGFR antibodies or sensitivity to targeted therapy in advanced colorectal cancer. Oncologist. 2016;21:1306–14.
Barry GS, Cheang MC, Chang HL, Kennecke HF. Genomic markers of panitumumab resistance including ERBB2/HER2 in a phase II study of KRAS wild-type (wt) metastatic colorectal cancer (mCRC). Oncotarget. 2016;7:18953–64.
Network Cancer Genome Atlas. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7.
Siena S, Sartore-Bianchi A, Marsoni S, Hurwitz HI, McCall SJ, Penault-Llorca F, et al. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol. 2018;29:1108–19.
Seo AN, Kwak Y, Kim D-W, Kang S-B, Choe G, Kim WH, et al. HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression, Creighton C, editor. PLoS ONE. 2014;9:e98528.
Takegawa N, Yonesaka K. HER2 as an emerging oncotarget for colorectal cancer treatment after failure of anti-epidermal growth factor receptor therapy. Clin Colorectal Cancer. 2017;16:247–51.
Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 2015;34:157–64.
Arkhipov A, Shan Y, Kim ET, Dror RO, Shaw DE. Her2 activation mechanism reflects evolutionary preservation of asymmetric ectodomain dimers in the human EGFR family. eLife [Internet]. 2013;2. https://elifesciences.org/articles/00708. Accessed 3 Jan 2019.
Rakha EA, Pinder SE, Bartlett JMS, Ibrahim M, Starczynski J, Carder PJ, et al. Updated UK recommendations for HER2 assessment in breast cancer. J Clin Pathol. 2015;68:93–9.
Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–50.
Kim EK, Kim KA, Lee CY, Shim HS. The frequency and clinical impact of HER2 alterations in lung adenocarcinoma. St-Pierre Y, editor. PLoS ONE. 2017;12:e0171280.
Richman SD, Southward K, Chambers P, Cross D, Barrett J, Hemmings G, et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol. 2016;238:562–70.
Lee W-S, Park YH, Lee JN, Baek J-H, Lee T-H, Ha SY. Comparison of HER2 expression between primary colorectal cancer and their corresponding metastases. Cancer Med. 2014;3:674–80.
Ramanathan RK, Hwang JJ, Zamboni WC, Sinicrope FA, Safran H, Wong MK, et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Investig. 2004;22:858–65.
Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1:508–23.
Jeong JH, Kim J, Hong YS, Kim D, Kim JE, Kim SY, et al. HER2 Amplification and Cetuximab Efficacy in Patients With Metastatic Colorectal Cancer Harboring Wild-type RAS and BRAF. Clin Colorectal Cancer. 2017;16:e147–e152152.
Nam SK, Yun S, Koh J, Kwak Y, Seo AN, Park KU, et al. BRAF, PIK3CA, and HER2 oncogenic alterations according to KRAS mutation status in advanced colorectal cancers with distant metastasis. PLoS ONE. 2016;11:e0151865.
Martin V, Landi L, Molinari F, Fountzilas G, Geva R, Riva A, et al. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer. 2013;108:668–75.
Valtorta E, Martino C, Sartore-Bianchi A, Penaullt-Llorca F, Viale G, Risio M, et al. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol. 2015;28:1481–91.
Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738–46.
Ingold Heppner B, Behrens H-M, Balschun K, Haag J, Krüger S, Becker T, et al. HER2/neu testing in primary colorectal carcinoma. Br J Cancer. 2014;111:1977–84.
Shabbir A, Mirza T, Khalid AB, Qureshi MA, Asim SA. Frequency of Her2/neu expression in colorectal adenocarcinoma: a study from developing South Asian Country. BMC Cancer [Internet]. 2016;16. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2912-y. Accessed 7 Jan 2019.
Blok EJ, Kuppen PJ, van Leeuwen JE, Sier CF. Cytoplasmic overexpression of HER2: a key factor in colorectal cancer. Clin Med Insights Oncol. 2013;7:41–51.
Half E, Broaddus R, Danenberg KD, Danenberg PV, Ayers GD, Sinicrope FA. HER-2 receptor expression, localization, and activation in colorectal cancer cell lines and human tumors. Int J Cancer. 2004;108:540–8.
Kavanagh DO, Chambers G, O’ Grady L, Barry KM, Waldron RP, Bennani F, et al. Is overexpression of HER-2 a predictor of prognosis in colorectal cancer? BMC Cancer [Internet]. 2009;9. https://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-9-1. Accessed 11 Jan 2019.
Marx AH, Burandt EC, Choschzick M, Simon R, Yekebas E, Kaifi JT, et al. Heterogenous high-level HER-2 amplification in a small subset of colorectal cancers. Hum Pathol. 2010;41:1577–85.
Ooi A, Takehana T, Li X, Suzuki S, Kunitomo K, Iino H, et al. Protein overexpression and gene amplification of HER-2 and EGFR in colorectal cancers: an immunohistochemical and fluorescent in situ hybridization study. Mod Pathol. 2004;17:895–904.
Osako T, Miyahara M, Uchino S, Inomata M, Kitano S, Kobayashi M. Immunohistochemical study of c-erbB-2 protein in colorectal cancer and the correlation with patient survival. Oncology. 1998;55:548–55.
Buhmeida A, Assidi M, Al-Maghrabi J, Dallol A, Sibiany A, Al-Ahwal M, et al. Membranous or cytoplasmic HER2 expression in colorectal carcinoma: evaluation of prognostic value using both IHC and BDISH. Cancer Investig. 2018;36:129–40.
Modest DP, Stintzing S, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, et al. Exploring the effect of primary tumor sidedness on therapeutic efficacy across treatment lines in patients with metastatic colorectal cancer: analysis of FIRE-3 (AIOKRK0306). Oncotarget [Internet]. 2017;8. https://www.oncotarget.com/fulltext/22396. Accessed 11 Jan 2019.
Yang SY, Cho MS, Kim NK. Difference between right-sided and left-sided colorectal cancers: from embryology to molecular subtype. Expert Rev Anticancer Ther. 2018;18:351–8.
Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients With RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3(2):194–201.
Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 2017;3(2):211–9.
Arnold D, Lueza B, Douillard J-Y, Peeters M, Lenz H-J, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713–29.
Missiaglia E, Jacobs B, D’Ario G, Di Narzo AF, Soneson C, Budinska E, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001.
Salem ME, Weinberg BA, Xiu J, El-Deiry WS, Hwang JJ, Gatalica Z, et al. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget. 2017;8:86356–68.
Ross JS, Fakih M, Ali SM, Elvin JA, Schrock AB, Suh J, et al. Targeting HER2 in colorectal cancer: the landscape of amplification and short variant mutations in ERBB2 and ERBB3: ERBB2 and ERBB3 in CRC. Cancer. 2018;124:1358–73.
Shimada Y, Yagi R, Kameyama H, Nagahashi M, Ichikawa H, Tajima Y, et al. Utility of comprehensive genomic sequencing for detecting HER2-positive colorectal cancer. Hum Pathol. 2017;66:1–9.
Siena S, Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, et al. Abstract CT005: final results of the HERACLES trial in HER2-amplified colorectal cancer. Cancer Res. 2017;77:CT005.
Normanno N, Cervantes A, Ciardiello F, De Luca A, Pinto C. The liquid biopsy in the management of colorectal cancer patients: current applications and future scenarios. Cancer Treat Rev. 2018;70:1–8.
Castro-Giner F, Gkountela S, Donato C, Alborelli I, Quagliata L, Ng C, et al. Cancer diagnosis using a liquid biopsy: challenges and expectations. Diagnostics. 2018;8:31.
Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:795–801.
Takegawa N, Yonesaka K, Sakai K, Ueda H, Watanabe S, Nonagase Y, et al. HER2 genomic amplification in circulating tumor DNA from patients with cetuximab-resistant colorectal cancer. Oncotarget [Internet]. 2016;7. https://www.oncotarget.com/fulltext/6498. Accessed 11 Jan 2019.
Kapitanović S, Radosević S, Kapitanović M, Andelinović S, Ferencić Z, Tavassoli M, et al. The expression of p185(HER-2/neu) correlates with the stage of disease and survival in colorectal cancer. Gastroenterology. 1997;112:1103–13.
McKay JA, Loane JF, Ross VG, Ameyaw M-M, Murray GI, Cassidy J, et al. c-erbB-2 is not a major factor in the development of colorectal cancer. Br J Cancer. 2002;86:568–73.
Song Z, Deng Y, Zhuang K, Li A, Liu S. Immunohistochemical results of HER2/neu protein expression assessed by rabbit monoclonal antibodies SP3 and 4B5 in colorectal carcinomas. Int J Clin Exp Pathol. 2014;7:4454–60.
Tol J, Dijkstra JR, Klomp M, Teerenstra S, Dommerholt M, Vink-Börger ME, et al. Markers for EGFR pathway activation as predictor of outcome in metastatic colorectal cancer patients treated with or without cetuximab. Eur J Cancer. 2010;46:1997–2009.
Laurent-Puig P, Balogoun R, Cayre A, Le Malicot K, Tabernero J, Mini E, et al. ERBB2 alterations a new prognostic biomarker in stage III colon cancer from a FOLFOX based adjuvant trial (PETACC8). Ann Oncol [Internet]. 2016;27. https://academic.oup.com/annonc/article/doi/10.1093/annonc/mdw370.08/2799198/ERBB2-alterations-a-new-prognostic-biomarker-in. Accessed 11 Jan 2019.
Sawada K, Nakamura Y, Yamanaka T, Kuboki Y, Yamaguchi D, Yuki S, et al. Prognostic and predictive value of HER2 amplification in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2018;17:198–205.
Kavuri SM, Jain N, Galimi F, Cottino F, Leto SM, Migliardi G, et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015;5:832–41.
Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3:99ra86.
Raghav KPS, et al. HER2 amplification as a negative predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. J Clin Oncol. 2016. https://doi.org/10.1200/JCO.2016.34.15_suppl.3517.
Zhao B, Wang L, Qiu H, Zhang M, Sun L, Peng P, et al. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget. 2017;8:3980–4000.
Ciardiello F, Normanno N. HER2 signaling and resistance to the anti-EGFR monoclonal antibody cetuximab: a further step toward personalized medicine for patients with colorectal cancer. Cancer Discov. 2011;1:472–4.
Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii1–iii9.
Kenny LM, Lam EW-F. Review: lapatinib in metastatic colorectal cancer-another strategy for disease control? Clin Adv Hematol Oncol. 2011;9:500–1.
Rubinson DA, Hochster HS, Ryan DP, Wolpin BM, McCleary NJ, Abrams TA, et al. Multi-drug inhibition of the HER pathway in metastatic colorectal cancer: results of a phase I study of pertuzumab plus cetuximab in cetuximab-refractory patients. Investig New Drugs. 2014;32:113–22.
Deeken JF, Wang H, Subramaniam D, He AR, Hwang J, Marshall JL, et al. A phase 1 study of cetuximab and lapatinib in patients with advanced solid tumor malignancies. Cancer. 2015;121:1645–53.
LaBonte MJ, Wilson PM, Fazzone W, Russell J, Louie SG, El-Khoueiry A, et al. The dual EGFR/HER2 inhibitor lapatinib synergistically enhances the antitumor activity of the histone deacetylase inhibitor panobinostat in colorectal cancer models. Cancer Res. 2011;71:3635–48.
Leto SM, Sassi F, Catalano I, Torri V, Migliardi G, Zanella ER, et al. Sustained inhibition of HER3 and EGFR is necessary to induce regression of HER2-amplified gastrointestinal carcinomas. Clin Cancer Res. 2015;21:5519–31.
Assenat E, Azria D, Mollevi C, Guimbaud R, Tubiana-Mathieu N, Smith D, et al. Dual targeting of HER1/EGFR and HER2 with cetuximab and trastuzumab in patients with metastatic pancreatic cancer after gemcitabine failure: results of the “THERAPY”phase 1–2 trial. Oncotarget. 2015;6:12796–808.
Larbouret C, Gaborit N, Chardès T, Coelho M, Campigna E, Bascoul-Mollevi C, et al. In pancreatic carcinoma, dual EGFR/HER2 targeting with cetuximab/trastuzumab is more effective than treatment with trastuzumab/erlotinib or lapatinib alone: implication of receptors’ down-regulation and dimers’ disruption. Neoplasia. 2012;14:121–30.
Martinelli E, Troiani T, Sforza V, Martini G, Cardone C, Vitiello PP, et al. Sequential HER2 blockade as effective therapy in chemorefractory, HER2 gene-amplified, RAS wild-type, metastatic colorectal cancer: learning from a clinical case. ESMO Open. 2018;3:e000299.
Gluck WL, Martin JC, Edenfield WJ, Chung KY, Arguello D. Prolonged response of widely metastatic HER2-positive colon cancer to trastuzumab therapy. Colorectal Cancer. 2017;6:57–61.
Parikh A, Atreya C, Korn WM, Venook AP. Prolonged response to HER2-directed therapy in a patient with HER2-amplified, rapidly progressive metastatic colorectal cancer. J Natl Compr Cancer Netw. 2017;15:3–8.
Kadowaki S. A triplet combination with irinotecan, oxaliplatin, continuous infusion 5-fluorouracil and leucovorin (FOLFOXIRI) plus cetuximab as first-line treatment in RAS wild-type, metastatic colorectal cancer: a phase 1 dose finding study. J Clin Oncol. 2018;suppl 4S:abstr 856.
Raghav KPS, et al. Detection and description of ERBB2 amplification using circulating cell free tumor DNA (ctDNA) genomic analysis in metastatic colorectal cancer (mCRC). J Clin Oncol. 2018;suppl 4S:abstr 661.
Acknowledgements
We would like to thank Dr. Anuradha Nalli and Dr. Amit Bhat from Indegene, Bangalore, for providing medical writing support and technical assistance in the preparation of this manuscript, as funded by Merck Serono China, an affiliate of Merck KGaA, Darmstadt, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The manuscript does not contain clinical studies or patient data.
Informed consent
Informed consent is not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, G., He, Y., Sun, Y. et al. Prevalence, prognosis and predictive status of HER2 amplification in anti-EGFR-resistant metastatic colorectal cancer. Clin Transl Oncol 22, 813–822 (2020). https://doi.org/10.1007/s12094-019-02213-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02213-9