Abstract
Purpose
This study aimed at investigating the efficacy of percutaneous transhepatic biliary stenting (PTBS) combined with 125I seeds intracavitary irradiation in the treatment of extrahepatic cholangiocarcinoma (EHC) and to preliminarily explore the prognostic values of inflammation-based scores in these patients.
Methods
A total of 113 clinically/pathologically diagnosed cases of EHC who received PTBS combined with 125I seeds implantation were retrospectively analyzed. The postoperative changes of clinical symptoms and serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total serum bilirubin (TBIL), direct bilirubin (DBIL), and albumin (ALB) were observed. Preoperative clinical data were extracted to calculate inflammation-based scores, including systemic immune-inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), and platelets-to-lymphocyte ratio (PLR). Kaplan–Meier survival curves and Cox regression analyses were used to evaluate the prognostic significance of inflammation-based scores.
Results
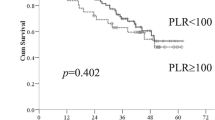
After operation, clinical symptoms such as jaundice and fever significantly improved in all patients. At 1 month and 3 months postoperatively, serum levels of ALT, AST, ALP, TBIL, and DBIL significantly reduced, and ALB significantly increased, compared with preoperative values. The median survival time of the patients was 12 months and the 1-year survival rate was 56.8%. Univariate analysis revealed that factors related to overall survival were CA19-9, TBIL, ALB, SII, and NLR. Multivariate analysis further identified SII and NLR as independent prognostic models.
Conclusion
The combination of PTBS and 125I seeds intracavitary irradiation is an effective palliative treatment for advanced EHC. Elevated SII and NLR can be used to predict poor survival.





Similar content being viewed by others
References
Cai Y, Cheng N, Ye H, Li F, Song P, Tang W. The current management of cholangiocarcinoma: a comparison of current guidelines. Biosci Trends. 2016;10:92–102.
Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma—evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2017;15:95–111.
Moole H, Tathireddy H, Dharmapuri S, Moole V, Boddireddy R, Yedama P, et al. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: a systematic review and meta-analysis. World J Gastroenterol. 2017;23:1278–88.
Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713–24.
Indar AA, Lobo DN, Gilliam AD, Gregson R, Davidson I, Whittaker S, et al. Percutaneous biliary metal wall stenting in malignant obstructive jaundice. Eur J Gastroenterol Hepatol. 2003;15:915–9.
Wang T, Liu S, Zheng YB, Song XP, Sun BL, Jiang WJ, et al. Clinical study on using 125I seeds articles combined with biliary stent implantation in the treatment of malignant obstructive jaundice. Anticancer Res. 2017;37:4649–53.
Tan Y, Zhu JY, Qiu BA, Xia NX, Wang JH. Percutaneous biliary stenting combined with radiotherapy as a treatment for unresectable hilar cholangiocarcinoma. Oncol Lett. 2015;10:2537–42.
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–71.
Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125:3347–55.
Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–503.
Qi X, Li J, Deng H, Li H, Su C, Guo X. Neutrophil-to-lymphocyte ratio for the prognostic assessment of hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. Oncotarget. 2016;7:45283–301.
Pang Q, Zhang LQ, Wang RT, Bi JB, Zhang JY, Qu K, et al. Platelet to lymphocyte ratio as a novel prognostic tool for gallbladder carcinoma. World J Gastroenterol. 2015;21:6675–83.
Anderson LL, Hsin MK, Ing-Yuan D. Clinical dosimetry with 125-I. In: George FW, editor. Modern interstitial and intracavitary radiation cancer management. New York: Masson; 1981. p. 9–15.
Mowbray NG, Griffith D, Hammoda M, Shingler G, Kambal A, Al-Sarireh B. A meta-analysis of the utility of the neutrophil-to-lymphocyte ratio in predicting survival after pancreatic cancer resection. HPB (Oxford). 2018;20:379–84.
Lee SH, Park SW. Inflammation and cancer development in pancreatic and biliary tract cancer. Korean J Gastroenterol. 2015;66:325–39.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4.
Jin H, Pang Q, Liu H, Li Z, Wang Y, Lu Y, et al. Prognostic value of inflammation-based markers in patients with recurrent malignant obstructive jaundice treated by reimplantation of biliary metal stents: a retrospective observational study. Medicine (Baltimore). 2017;96:e5895.
Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–29.
Cidon EU. Resectable cholangiocarcinoma: reviewing the role of adjuvant strategies. Clin Med Insights Oncol. 2016;10:43–8.
Squadroni M, Tondulli L, Gatta G, Mosconi S, Beretta G, Labianca R. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2017;116:11–31.
Witzigmann H, Lang H, Lauer H. Guidelines for palliative surgery of cholangiocarcinoma. HPB (Oxford). 2008;10:154–60.
Boulay BR, Birg A. Malignant biliary obstruction: from palliation to treatment. World J Gastrointest Oncol. 2016;8:498–508.
Barkun AN, Adam V, Martel M, AlNaamani K, Moses PL. Partially covered self-expandable metal stents versus polyethylene stents for malignant biliary obstruction: a cost-effectiveness analysis. Can J Gastroenterol Hepatol. 2015;29:377–83.
Lee TH, Lee SJ, Moon JH, Park SH. Technical tips and issues of biliary stenting, focusing on malignant hilar obstruction. Minerva Gastroenterol Dietol. 2014;60:135–49.
Mattiucci GC, Autorino R, Tringali A, Perri V, Balducci M, Deodato F, et al. A Phase I study of high-dose-rate intraluminal brachytherapy as palliative treatment in extrahepatic biliary tract cancer. Brachytherapy. 2015;14:401–4.
Popescu CC, Wise J, Sowards K, Meigooni AS, Ibbott GS. Dosimetric characteristics of the Pharma Seed model BT-125-I source. Med Phys. 2000;27:2174–81.
Qiu H, Ji J, Shao Z, Wang J, Ma G, Zhang L, et al. The efficacy and safety of iodine-125 brachytherapy combined with chemotherapy in treatment of advanced lung cancer: a meta-analysis. J Coll Physicians Surg Pak. 2017;27:237–45.
Du E, Wang L, Li CY, Zhang CW, Qu YC, Liu RL, et al. Analysis of immune status after iodine-125 permanent brachytherapy in prostate cancer. Onco Targets Ther. 2017;10:2561–7.
Kubo M, Satoh T, Ishiyama H, Tabata KI, Tsumura H, Komori S, et al. Enhanced activated T cell subsets in prostate cancer patients receiving iodine-125 low-dose-rate prostate brachytherapy. Oncol Rep. 2018;39:417–24.
Zhu HD, Guo JH, Zhu GY, He SC, Fang W, Deng G, et al. A novel biliary stent loaded with (125)I seeds in patients with malignant biliary obstruction: preliminary results versus a conventional biliary stent. J Hepatol. 2012;56:1104–11.
Shinohara ET, Guo M, Mitra N, Metz JM. Brachytherapy in the treatment of cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2010;78:722–8.
Nag S, DeHaan M, Scruggs G, Mayr N, Martin EW. Long-term follow-up of patients of intrahepatic malignancies treated with iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys. 2006;64:736–44.
Okuno M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, et al. Appraisal of inflammation-based prognostic scores in patients with unresectable perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2016;23:636–42.
Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. https://doi.org/10.1093/jnci/dju124.
Kitano Y, Yamashita YI, Yamamura K, Arima K, Kaida T, Miyata T, et al. Effects of preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios on survival in patients with extrahepatic cholangiocarcinoma. Anticancer Res. 2017;37:3229–37.
Suzuki S, Kajiyama H, Shimoda M, Shimazaki J, Koike N, Harada N. Prognostic factors associated with preoperative clinicophysiological outcomes of distal cholangiocarcinoma. Dig Surg. 2017;34:476–82.
Gerhardt T, Milz S, Schepke M, Feldmann G, Wolff M, Sauerbruch T, et al. C-reactive protein is a prognostic indicator in patients with perihilar cholangiocarcinoma. World J Gastroenterol. 2006;12:5495–500.
Oshiro Y, Sasaki R, Fukunaga K, Kondo T, Oda T, Takahashi H, et al. Inflammation-based prognostic score is a useful predictor of postoperative outcome in patients with extrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2013;20:389–95.
Carr BI, Cavallini A, D’Alessandro R, Refolo MG, Lippolis C, Mazzocca A, et al. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer. 2014;14:43.
Olsson AK, Cedervall J. The pro-inflammatory role of platelets in cancer. Platelets. 2018;29:569–73.
Plantureux L, Crescence L, Dignat-George F, Panicot-Dubois L, Dubois C. Effects of platelets on cancer progression. Thromb Res. 2018;164(Suppl 1):S40–7.
Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887–96.
Man YG, Stojadinovic A, Mason J, Avital I, Bilchik A, Bruecher B, et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer. 2013;4:84–95.
Iwase R, Shiba H, Haruki K, Fujiwara Y, Furukawa K, Futagawa Y, et al. Post-operative lymphocyte count may predict the outcome of radical resection for gallbladder carcinoma. Anticancer Res. 2013;33:3439–44.
Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103.
Fidler IJ, Schroit AJ. Recognition and destruction of neoplastic cells by activated macrophages: discrimination of altered self. Biochim Biophys Acta. 1988;948:151–73.
Acknowledgements
This work was supported by the Science and Technology Innovation Fund project of Anhui Province (Grant no. 1501041155).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declared no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All patients signed informed consent prior to treatment.
Rights and permissions
About this article
Cite this article
Hu, X., Pang, Q., Liu, H. et al. Inflammation-based prognostic scores in patients with extrahepatic bile duct lesions treated by percutaneous transhepatic biliary stenting combined with 125I seeds intracavitary irradiation. Clin Transl Oncol 21, 665–673 (2019). https://doi.org/10.1007/s12094-018-1969-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1969-2