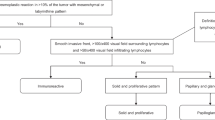
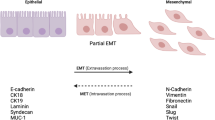
Abstract
Endometrial cancer (EC) is the most common gynecologic malignancy of the female genital tract and the fourth most common neoplasia in women. In EC, myometrial invasion is considered one of the most important prognostic factors. For this process to occur, epithelial tumor cells need to undergo an epithelial to mesenchymal transition (EMT), either transiently or stably, and to differing degrees. This process has been extensively described in other types of cancer but has been poorly studied in EC. In this review, several features of EMT and the main molecular pathways responsible for triggering this process are investigated in relation to EC. The most common hallmarks of EMT have been found in EC, either at the level of E-cadherin loss or at the induction of its repressors, as well as other molecular alterations consistent with the mesenchymal phenotype-like L1CAM and BMI-1 up-regulation. Pathways including progesterone receptor, TGFβ, ETV5 and microRNAs are deeply related to the EMT process in EC.


Similar content being viewed by others
References
Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15:10–17
Zannoni GF, Scambia G, Gallo D (2011) The dualistic model of endometrial cancer: the challenge of classifying grade 3 endometrioid carcinoma. Gynecol Oncol. doi:10.1016/j.ygyno.2011.09.036
Llauradó M et al (2011) Molecular bases of endometrial cancer: new roles for new actors in the diagnosis and the therapy of the disease. Mol Cell Endocrinol. doi:10.1016/j.mce.2011.10.003
Amant F et al (2005) Endometrial cancer. Lancet 366:491–505
Yeramian A et al (2012) Endometrial carcinoma: molecular alterations involved in tumor development and progression. Oncogene. doi:10.1038/onc.2012.76
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Abal M et al (2007) Molecular determinants of invasion in endometrial cancer. Clin Transl Oncol 9:272–277
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Moreno-Bueno G, Portillo F, Cano A (2008) Transcriptional regulation of cell polarity in EMT and cancer. Oncogene 27:6958–6969
Yang J, Weinberg RA (2008) Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14:818–829
Thiery JP, Morgan M (2004) Breast cancer progression with a Twist. Nat Med 10:777–778
Kang Y, Massague J (2004) Epithelial–mesenchymal transitions: twist in development and metastasis. Cell 118:277–279
Mueller MM, Fusenig NE (2004) Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4:839–849
Bhowmick NA, Neilson EG, Moses HL (2004) Stromal fibroblasts in cancer initiation and progression. Nature 432:332–337
Barrallo-Gimeno A, Nieto MA (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132:3151–3161
De Craene B, van Roy F, Berx G (2005) Unraveling signaling cascades for the Snail family of transcription factors. Cell Signal 17:535–547
Huber M, Kraut N, Beug H (2005) Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr Opin Cell Biol 17:548–558
Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol 7:131–142
Micalizzi D, Farabaugh S, Ford H (2010) Epithelial–mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 15:117–134
Hurt EM, Saykally JN, Anose BM, Kalli KR, Sanders MM (2008) Expression of the ZEB1 (deltaEF1) transcription factor in human: additional insights. Mol Cell Biochem 318:89–99
Singh M et al (2008) ZEB1 expression in type I vs type II endometrial cancers: a marker of aggressive disease. Mod Pathol 21:912–923
Kyo S et al (2006) High Twist expression is involved in infiltrative endometrial cancer and affects patient survival. Hum Pathol 37:431–438
Blechschmidt K et al (2007) The E-cadherin repressor snail plays a role in tumor progression of endometrioid adenocarcinomas. Diagn Mol Pathol 16:222–228
Shih H-C et al (2004) Immunohistochemical expression of E-cadherin and beta-catenin in the normal and malignant human endometrium: an inverse correlation between E-cadherin and nuclear beta-catenin expression. Anticancer Res 24:3843–3850
Scholten AN, Aliredjo R, Creutzberg CL, Smit VTHB (2006) Combined E-cadherin, alpha-catenin, and beta-catenin expression is a favorable prognostic factor in endometrial carcinoma. Int J Gynecol Cancer 16:1379–1385
Sakuragi N et al (1994) Decreased E-cadherin expression in endometrial carcinoma is associated with tumor dedifferentiation and deep myometrial invasion. Gynecol Oncol 53:183–189
Moreno-Bueno G et al (2003) Abnormalities of E- and P-cadherin and catenin (beta-, gamma-catenin, and p120ctn) expression in endometrial cancer and endometrial atypical hyperplasia. J Pathol 199:471–478
Stefansson IM, Salvesen HB, Akslen LA (2004) Prognostic impact of alterations in P-cadherin expression and related cell adhesion markers in endometrial cancer. J Clin Oncol 22:1242–1252
Mell LK et al (2004) Prognostic significance of E-cadherin protein expression in pathological stage I–III endometrial cancer. Clin Cancer Res 10:5546–5553
Leblanc M et al (2001) Alteration of CD44 and cadherins expression: possible association with augmented aggressiveness and invasiveness of endometrial carcinoma. Virchows Arch 438:78–85
Huszar M et al (2010) Up-regulation of L1CAM is linked to loss of hormone receptors and E-cadherin in aggressive subtypes of endometrial carcinomas. J Pathol 220:551–561
Pfeifer M et al (2010) L1CAM expression in endometrial carcinomas is regulated by usage of two different promoter regions. BMC Mol Biol 11:64
Lee H et al(2012) Immunohistochemical analysis of polycomb group protein expression in advanced gastric cancer. Hum Pathol. doi:10.1016/j.humpath.2011.12.019
Vonlanthen S et al (2001) The bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A-ARF locus expression. Br J Cancer 84:1372–1376
Guo B-H et al (2011) Bmi-1 promotes invasion and metastasis, and its elevated expression is correlated with an advanced stage of breast cancer. Mol. Cancer 10:10
Glinsky GV, Berezovska O, Glinskii AB (2005) Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest 115:1503–1521
Zhang F, Sui L, Xin T (2008) Correlations of BMI-1 expression and telomerase activity in ovarian cancer tissues. Exp Oncol 30:70–74
Honig A et al (2010) Overexpression of polycomb protein BMI-1 in human specimens of breast, ovarian, endometrial and cervical cancer. Anticancer Res 30:1559–1564
Dong P et al (2011) MicroRNA-194 inhibits epithelial to mesenchymal transition of endometrial cancer cells by targeting oncogene BMI-1. Mol Cancer 10:99
Stewart CJR, Little L (2009) Immunophenotypic features of MELF pattern invasion in endometrial adenocarcinoma: evidence for epithelial–mesenchymal transition. Histopathology 55:91–101
Hanekamp EE et al (2005) Differences in invasive capacity of endometrial cancer cell lines expressing different progesterone receptor isotypes: possible involvement of cadherins. J Soc Gynecol Invest 12:278–284
Dai D, Wolf DM, Litman ES, White MJ, Leslie KK (2002) Progesterone inhibits human endometrial cancer cell growth and invasiveness: down-regulation of cellular adhesion molecules through progesterone B receptors. Cancer Res 62:881–886
Hanekamp EE et al (2002) Loss of progesterone receptor may lead to an invasive phenotype in human endometrial cancer. Eur J Cancer 38(Suppl 6):S71–S72
Van der Horst PH et al (2012) Progesterone inhibits epithelial-to-mesenchymal transition in endometrial cancer. PLoS One 7(1):e30840
Fujita N et al (2003) MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell 113:207–219
Dandachi N et al (2001) Co-expression of tenascin-C and vimentin in human breast cancer cells indicates phenotypic transdifferentiation during tumour progression: correlation with histopathological parameters, hormone receptors, and oncoproteins. J Pathol 193:181–189
Ehrlich CE, Young PC, Stehman FB, Sutton GP, Alford WM (1988) Steroid receptors and clinical outcome in patients with adenocarcinoma of the endometrium. Am J Obstet Gynecol 158:796–807
Jeon Y-T et al (2006) Steroid receptor expressions in endometrial cancer: clinical significance and epidemiological implication. Cancer Lett 239:198–204
Thigpen JT et al (1999) Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol 17:1736–1744
Hanekamp EE et al (2003) Consequences of loss of progesterone receptor expression in development of invasive endometrial cancer. Clin Cancer Res 9:4190–4199
Wang Y et al (2009) Progesterone inhibition of Wnt/beta-catenin signaling in normal endometrium and endometrial cancer. Clin Cancer Res 15:5784–5793
Massagué J (2008) TGFbeta in Cancer. Cell 134:215–230
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signaling. Nature 425:577–584
Parekh TV et al (2002) Transforming growth factor beta signaling is disabled early in human endometrial carcinogenesis concomitant with loss of growth inhibition. Cancer Res 62:2778–2790
Muinelo-Romay L et al (2011) High-risk endometrial carcinoma profiling identifies TGF-β1 as a key factor in the initiation of tumor invasion. Mol Cancer Ther 10:1357–1366
Lei X, Wang L, Yang J, Sun L-Z (2009) TGFbeta signaling supports survival and metastasis of endometrial cancer cells. Cancer Manag Res 2009:15–24
de Launoit Y et al (2006) The Ets transcription factors of the PEA3 group: transcriptional regulators in metastasis. Biochim Biophys Acta 1766:79–87
Graves BJ, Petersen JM (1998) Specificity within the ets family of transcription factors. Adv Cancer Res 75:1–55
Planaguma J et al (2005) Up-regulation of ERM/ETV5 correlates with the degree of myometrial infiltration in endometrioid endometrial carcinoma. J Pathol 207:422–429
Llauradó M et al (2012) ETV5 transcription factor is overexpressed in ovarian cancer and regulates cell adhesion in ovarian cancer cells. Int J Cancer 130:1532–1543
Colas E et al (2012) ETV5 cooperates with LPP as a sensor of extracellular signals and promotes EMT in endometrial carcinomas. Oncogene. doi:10.1038/onc.2011.632
Monge M et al (2007) ERM/ETV5 up-regulation plays a role during myometrial infiltration through matrix metalloproteinase-2 activation in endometrial cancer. Cancer Res 67:6753–6759
Monge M et al (2009) Proteomic approach to ETV5 during endometrial carcinoma invasion reveals a link to oxidative stress. Carcinogenesis 30:1288–1297
Monge M et al (2009) Subtractive proteomic approach to the endometrial carcinoma invasion front. J Proteome Res 8:4676–4684
Ebert MS, Sharp PA (2012) Roles for MicroRNAs in conferring robustness to biological processes. Cell 149:515–524
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Lujambio A, Lowe SW (2012) The microcosmos of cancer. Nature 482:347–355
Castilla MA et al (2011) Micro-RNA signature of the epithelial–mesenchymal transition in endometrial carcinosarcoma. J Pathol 223:72–80
Kong W et al (2008) MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol 28:6773–6784
Howe EN, Cochrane DR, Richer JK (2011) Targets of miR-200c mediate suppression of cell motility and anoikis resistance. Breast Cancer Res 13:R45
Cano A, Nieto MA (2008) Non-coding RNAs take centre stage in epithelial-to-mesenchymal transition. Trends Cell Biol 18:357–359
Acknowledgments
The authors would like to thank Lisa Piccione for correction of the manuscript. This work has been supported by the Spanish Ministry of Science and Innovation (SAF 2005-06771; SAF 2008-03996; SAF 2010-10635-E; SAF2011-26548), CENIT Program (CENIT/01/2006) and RTICC Program (RTICC RD06/0020/0058 and RD06/0020/1034), the Catalan Institute of Health and the Department of Universities and Research, Catalan Government (2009SGR00487, 2005SGR00553), the ACCIO Program (RDITSCON07-1-0001), the Foundation La Marato de TV3 (Grant 050431), the IV Grant Fundació Santiago Dexeus Font for Clinical Investigation Projects 2009, the National Programme of Biotechnology (FIT-010000-2007-26), the Asociación Española Contra el Cáncer (AECC) and the European Commission Program Fondo Europeo de Desarrollo Regional (FEDER).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colas, E., Pedrola, N., Devis, L. et al. The EMT signaling pathways in endometrial carcinoma. Clin Transl Oncol 14, 715–720 (2012). https://doi.org/10.1007/s12094-012-0866-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-012-0866-3