Abstract
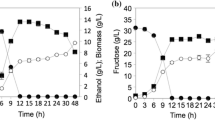
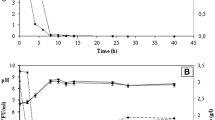
The search for promising yeasts that surpass the fermentative capacity of commercial strains, such as Saccharomyces cerevisiae CAT-1, is of great importance for industrial ethanol processes in the world. Two yeasts, Pichia kudriavzevii BB2 and Saccharomyces cerevisiae BB9, were evaluated in comparison to the industrial yeast S. cerevisiae CAT-1. The objective was to evaluate the performance profile of the three studied strains in terms of growth, substrate consumption, and metabolite formation, aiming to determine their behaviour in different media and pH conditions. The results showed that under cultivation conditions simulating the medium used in the industrial process (must at 22° Brix at pH 3.0) the highest ethanol productivity was 0.41 g L−1 h−1 for S. cerevisiae CAT-1, compared to 0.11 g L−1 h−1 and 0.16 g L−1 h−1 for P. kudriavzevii and S. cerevisiae BB2, respectively. S. cerevisiae CAT-1 produced three times more ethanol in must at pH 3.0 (28.30 g L−1) and in mineral medium at pH 3.0 (29.17 g L−1) and 5.0 (30.70 g L−1) when compared to the value obtained in sugarcane must pH 3.0 (9.89 g L−1). It was concluded that S. cerevisiae CAT-1 was not limited by the variation in pH in the mineral medium due to its nutritional composition, guaranteeing better performance of the yeast even in the presence of stressors. Only S. cerevisiae CAT-1 expressed he constitutive invertase enzyme, which is responsible for hydrolysing the sucrose contained in the must.



Similar content being viewed by others
References
Patel SKS, Kumar V, Mardina P, Li J, Lestari R, Kalia VC, Lee JK (2018) Methanol production from simulated biogas mixtures by co-immobilized Methylomonas methanica and Methylocella tundrae. Biores Technol 263:25–32
Kumar V, Patel SKS, Gupta RK, Otari SV, Gao H, Lee J, Zhang L (2019) Enhanced saccharification and fermentation of rice straw by reducing the concentration of phenolic compounds using an immobilized enzyme cocktail. Biotechnol J 302:1–8. https://doi.org/10.1002/biot.201800468
Patel SKS, Jeon MS, Gupta RK, Jeon Y, Kalia VC, Kim SC, Lee J-K (2019) Hierarchical macro-porous particles for efficient whole-cell immobilization: application in bioconversion of greenhouse gases to methanol. ACS Appl Mater Interfaces 11:18968–18977. https://doi.org/10.1021/acsami.9b03420
Patel SKS, Ray S, Prakash J, Wee JH, Kim S-Y, Lee J-K, Kalia VC (2019) Co-digestion of biowastes to enhance biological hydrogen process by defined mixed bacterial cultures. Indian J Microbiol. https://doi.org/10.1007/s12088-018-00777-8
Della-Bianca BE, Basso TO, Stambuk BU, Basso LC, Gombert AK (2013) What do we know about the yeast strains from the Brazilian fuel ethanol industry? Appl Microbiol Biotechnol 97:979–991. https://doi.org/10.1007/s00253-012-4631-x
Carvalho AL, Antunes CH, Freire F (2016) Economic-energy-environment analysis of prospective sugarcane bioethanol production in Brazil. Appl Energy 181:514–526. https://doi.org/10.1016/j.apenergy.2016.07.122
Raza G, Ali K, Hassan MA, Ashraf M, Kahan MT, Kahan IA (2019) Sugarcane as a Bioenergy Source. In: Kahan MT, Kahan IA (eds) Sugarcane biofuels: status, potential, and prospects of the sweet crop to fuel the world. Springer, Switzerland, pp 3–19
Bechara R, Gomez A, Saint-Antonin V, Schweitzer J-M, Maréchal F, Ensinas A (2018) Review of design works for the conversion of sugarcane to first and second-generation ethanol and electricity. Renew Sust Energy Rev 91:152–164. https://doi.org/10.1016/j.rser.2018.02.020
Carvalho DJ, Moretti RR, Colodette JL, Bizzo WA (2020) Assessment of the self-sustained energy generation of an integrated first and second generation ethanol production from sugarcane through the characterization of the hydrolysis process residues. Energy Convers Manag 203:112267. https://doi.org/10.1016/j.enconman.2019.112267
Wang L, Xin-qing Zhao, Xue C, Feng-Wu Bai (2013) Impact of osmotic stress and ethanol inhibition in yeast cells on process oscillation associated with continuous very-high-gravity ethanol fermentation. Biotechnol Biofuels 6:133. https://doi.org/10.1186/1754-6834-6-133
Dangi AK, Dubey KK, Shukla P (2017) Strategies to improve Saccharomyces cerevisiae: technological advancements and evolutionary engineering. Indian J Microbiol 57:378–386. https://doi.org/10.1007/s12088-017-0679-8
Camargo JZ, Nascimento VM, Stefanello I, Silva CAA, Gonçalves FA, Perdomo IC, Vilela DM, Simionatto S, Pereira RM, Paz MF, Leite RSR, Gelinski JMLN, Fonseca GG (2018) Biochemical evaluation, molecular characterization and identification of novel yeast strains isolated from Brazilian savannah fruits, chicken litter and a sugar and alcohol mill with biotechnological potential for biofuel and food industries. Biocatal Agric Biotechnol 16:390–399. https://doi.org/10.1016/j.bcab.2018.09.006
Silva RO, Batistote M, Cereda MP (2011) Wild strains of fermenting yeast isolated of sugar cane juice from an alcohol distillery from Mato Grosso, Brazil. J Biotechnol Biodivers 2:22–27. https://doi.org/10.1590/s1516-89132013000200001
Araque E, Parra C, Rodriguez M, Freer J, Baeza J (2008) Selection ofthermotolerant yeast strains Saccharomyces cerevisiae for bioethanol production. Enzyme Microbial Technol 43:120–123. https://doi.org/10.1016/j.enzmictec.2008.02.007
Lima UA, Aquarone E, Borzani W, Schmidell W (2001) Biotecnologia Industrial: processos Fermentativos e Enzimáticos. Blucher, São Paulo
Nascimento VM, Fonseca GG (2019) Effects of the carbon source and the interaction between carbon sources on the physiology of the industrial Saccharomyces cerevisiae CAT-1. Prep Biochem Biotechnol 11:1–8. https://doi.org/10.1080/10826068.2019.1703192
Fonseca GG, Carvalho NMB, Gombert AK (2013) Growth of the yeast Kluyveromyces marxianus CBS 6556 on different sugar combinations as sole carbon and energy source. App Microbiol Biotechnol 97:5055–5067. https://doi.org/10.1007/s00253-013-4748-6
Barbosa PMG, Morais TP, Silva CAA, Santos FRS, Garcia NFL, Fonseca GG, Leite RSR, Paz MF (2018) Biochemical characterization and evaluation of invertases produced from Saccharomyces cerevisiae CAT-1 and Rhodotorula mucilaginosa for the production of fructooligosaccharides. Prep Biochem Biotechnol 48:506–513. https://doi.org/10.1080/10826068.2018.1466155
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Patel SKS, Shanmugam R, Kalia VC, Lee J-K (2020) Methanol production by polymer-encapsulated methanotrophs from simulated biogas in the presence of methane vector. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.123022
Stephanopoulos GN, Aristidou AA, Nielsen J (1998) Metabolic engineering principles and methodologies. Academic Press, San Diego
Sankaran S, Qureshi MAA, Subbaiah S, Kavitha M (2017) Isolation and optimization of constitutively synthesized invertase, from Saccharomyces cerevisiae mutant type strain. Int J Appl Nat Sci. https://doi.org/10.13140/rg.2.2.17876.35207
Lin Z, Li W-H (2010) Expansion of hexose transporter genes was associated withthe evolution of aerobic fermentation in yeasts. Mol Biol Evol 28:131–142. https://doi.org/10.1093/molbev/msq184
Marques WL, Mans R, Marella ER, Cordeiro RL, van den Broek M, Daran J-MG, Pronk JT, Gombert AK, van Maris AJA (2017) Elimination of sucrose transport and hydrolysis in Saccharomyces cerevisiae: a platform strain for engineering sucrose metabolism. FEMS Yeast Res 9:1–17. https://doi.org/10.1093/femsyr/fox006
Stambuk BU, Dunn B, Alves SL Jr, Duval EH, Sherlock G (2009) Industrial fuel ethanol yeasts contain adaptive copy number changes in genes involved in vitamin B1 and B6 biosynthesis. Gen Res 19:2271–2278. https://doi.org/10.1101/gr.094276.109
Rogowska A, Pomastowski P, Złoch M, Railean-Plugaru V, Król A, Rafńska K, Szultka-Młyńska M, Buszewski B (2018) The infuence of diferent pH on the electrophoretic behaviour of Saccharomyces cerevisiae modifed by calcium ions. Sci Rep 8:7261. https://doi.org/10.1038/s41598-018-25024-4
Della-Bianca BE, Hulster E, Pronk JT, van Maris AJA, Gombert AK (2014) Physiology of the fuel ethanol strain Saccharomyces cerevisiae PE-2 at low pH indicates a context-dependent performance relevant for industrial applications. FEMS Yeast Res 14:1196–1205. https://doi.org/10.1111/1567-1364.12217
Marques WL, Raghavendran V, Stambuk BU, Gombert AK (2016) Sucrose and Saccharomyces cerevisiae: a relationship most sweet. FEMS Yeast Res 16:1–16. https://doi.org/10.1093/femsyr/fov107
Batistote M, Cardoso CAL, Ramos DD, Ernandes JR (2010) Desempenho de leveduras obtidas em indústrias de Mato Grosso do Sul na produção de etanol em mosto a base de cana de açúcar. Ciênc Nat 32:83–95. https://doi.org/10.5902/2179460x9487
Wu R, Chen D, Cao S, Lu Z, Huang J, Lu Q, Chen Y, Chen X, Guan N, Weia Y, Huang R (2020) Enhanced ethanol production from sugarcane molasses by industrially engineered Saccharomyces cerevisiae via replacement of the PHO4 gene. RSC Adv 10:2267–2276. https://doi.org/10.1039/c9ra08673k
Lopes ML, Paulillo SCL, Godoy A, Cherubin RA, Lorenzi MS, Giometti FHC, Bernardino CD, Neto HBA, Amorim HV (2016) Ethanol production in Brazil: a bridge between science and industry. Braz J Microbiol 47:64–76. https://doi.org/10.1016/j.bjm.2016.10.003
Fonseca GG, Gombert AK, Heinzle E, Wittmann C (2007) Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Res 7:422–435. https://doi.org/10.1111/j.1567-1364.2006.00192.x
Imura M, Nitta K, Iwakiri R, Matsuda F, Shimizu H, Fukusaki E (2020) Comparison of metabolic profiles of yeasts based on the difference of the Crabtree positive and negative. J Biosci Bioeng 129:52–58. https://doi.org/10.1016/j.jbiosc.2019.07.007
Traven A, Wong JMS, Xu D, Sopta M, Ingles CJ (2000) Interorganellar communication: altered nuclear gene expression profiles in a yeast mitochondrial DNA mutant. J Biol Chem 276:4020–4027. https://doi.org/10.1074/jbc.m006807200
Souza RB, Menezes JAS, Souza RFR, Dutra ED, Morais MA (2015) Mineral composition of the sugarcane juice and its influence on the ethanol fermentation. Appl Biochem Biotechnol 175:209–222. https://doi.org/10.1007/s12010-014-1258-7
Roca-Mesa H, Sendra S, Mas A, Beltran G, Torija M-J (2020) Nitrogen preferences during alcoholic fermentation of different Non-Saccharomyces yeasts of oenological interest. Microorganisms 8:157. https://doi.org/10.3390/microorganisms8020157
Zhang Q, Wu D, Lin Y, Wang X, Kong H, Tanaka S (2015) Substrate and product inhibition on yeast performance in ethanol fermentation. Energy Fuels 29:1019–1027. https://doi.org/10.1021/ef502349v
Basso TO, Kok S, Dario M, Espírito-Santo JCA, Muller G, Schlong PS, Silva CP, Tonso A, Daran J, Gombert AK, Maris AJAV, Pronk JT, Stambuk BU (2011) Engineering topology and kinetics of sucrose metabolism in Saccharomyces cerevisiae for improved ethanol yield. Metabol Eng 13:694–703. https://doi.org/10.1016/j.ymben.2011.09.005
Marques WL, Mans R, Marella ER, Cordeiro RL, Van Den Broek M, Daran JG, Pronk JT, Gombert AK, Van Maris JA (2017) Elimination of sucrose transport and hydrolysis in Saccharomyces cerevisiae: a platform strain for engineering sucrose metabolism. FEMS Yeast Res 17:1–11. https://doi.org/10.1093/femsyr/fox006
Li B, Liu H, Zhang Y, Kang T, Zhang L, Tong J, Xiao L, Zhang H (2013) Constitutive expression of cell wall invertase genes increases grain yield and starch content in maize. Plant Biotechnol J 11:1080–1091. https://doi.org/10.1111/pbi.12102
Babrzadeh F, Jalili R, Wang C, Shokralla S, Pierce S, Robinson-Mosher A, Nyren P, Shafer RW, Basso LC, Amorim HV, Oliveira AJ, Davis RW, Ronaghi M, Gharizadeh B, Stambuk BU (2012) Whole-genome sequencing of the efficient industrial fuel-ethanol fermentative Saccharomyces cerevisiae strain CAT-1. Mol Gen Genom 287:485–494. https://doi.org/10.1007/s00438-012-0695-7
Acknowledgements
The authors gratefully acknowledge the Brazilian research funding agencies: Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fernandes, A.M.O., Garcia, N.F.L., Fonseca, G.G. et al. Evaluation of the Fermentative Capacity of Saccharomyces cerevisiae CAT-1 and BB9 Strains and Pichia kudriavzevii BB2 at Simulated Industrial Conditions. Indian J Microbiol 60, 494–504 (2020). https://doi.org/10.1007/s12088-020-00891-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-020-00891-6