Abstract
Background
Hepatocellular carcinoma (HCC) is subclassified into five gross types, namely, vaguely nodular (VN), single nodular (SN), single nodular with extranodular growth (SNEG), confluent multinodular (CM), and infiltrative (INF) type. However, the pathological background underlying differences in biological behavior of different gross types of HCC remains unclear.
Methods
The histopathological features, clinical outcomes of HCC gross types, and their relationships with stemness-related marker status and fibrotic/hypoxic tumor microenvironment (TME) were evaluated in 266 resected HCCs. The stemness-related markers (CD24, CD44, CD133, SALL4, YAP1, K19 and EpCAM), fibrous tumor stroma (αSMA), and hypoxia (CAIX) were evaluated with immunohistochemistry.
Results
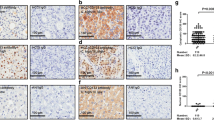
Poorer differentiation, reduced capsule formation, higher microvascular invasion, larger tumor size and larger area of necrosis were observed in order of VN-SN-SNEG-CM-INF type (p = 0.005 for all, linear-by-linear association). The expression of summed stemness-related markers and hypoxic/fibrotic TME showed an increasing trend in order of VN-SN-SNEG-CM-INF type (p < 0.005), and their expression well correlated with each other. INF type was found only in HCCs with hypoxic/fibrotic TME or high expression of stemness-related markers. CAIX expression and tumor necrosis ≥ 30% were independent prognostic markers for disease-specific survival. Early recurrence-free survival showed a significant difference based on gross types, revealing best outcome with VN type and worst outcome with INF type.
Conclusion
The marker expression of stemness-related and hypoxic/fibrotic TME of HCC showed an increasing trend in order of VN-SN-SNEG-CM-INF gross types, and their cross-talk may be involved in the determination of various gross-morphological features and their distinct biological behavior.




Similar content being viewed by others
References
Stewart BW, Wild C, WHO, International Agency for Research on Cancer. World cancer report 2014, Lyon, IARC Press, 2014
Eggel H. Ueber das primare carcinoma der leber. Beitr z Path Anat u z allgem Pathol 1901;30:506–604
Kanai T, Hirohashi S, Upton MP, et al. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer 1987;60:810–9
Hui AM, Takayama T, Sano K, et al. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol 2000;33:975–9
Shimada M, Rikimaru T, Hamatsu T, et al. The role of macroscopic classification in nodular-type hepatocellular carcinoma. Am J Surg 2001;182:177–82
Liver Cancer Study Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer. 2nd ed. Tokyo: Kanehara & Co.; 2003
Burt AD, Alves V, Bedossa P, et al. Data set for the reporting of intrahepatic cholangiocarcinoma, perihilar cholangiocarcinoma and hepatocellular carcinoma: recommendations from the International Collaboration on Cancer Reporting (ICCR). Histopathology. 2018;73:369–85.
Oishi N, Yamashita T, Kaneko S. Molecular biology of liver cancer stem cells. Liver Cancer 2014;3:71–84
Kim H, Choi GH, Na DC, et al. Human hepatocellular carcinomas with "Stemness"-related marker expression: keratin 19 expression and a poor prognosis. Hepatology 2011;54:1707–17
Kubo N, Araki K, Kuwano H, Shirabe K. Cancer-associated fibroblasts in hepatocellular carcinoma. World J Gastroenterol 2016;22:6841
Seok JY, Na DC, Woo HG, et al. A fibrous stromal component in hepatocellular carcinoma reveals a cholangiocarcinoma-like gene expression trait and epithelial-mesenchymal transition. Hepatology 2012;55:1776–86
Rhee H, Kim HY, Choi JH, et al. Keratin 19 expression in hepatocellular carcinoma is regulated by fibroblast-derived HGF via a MET-ERK1/2-AP1 and SP1 axis. Cancer Res 2018;78:1619–31
Rhee H, Nahm JH, Kim H, et al. Poor outcome of hepatocellular carcinoma with stemness marker under hypoxia: resistance to transarterial chemoembolization. Mod Pathol 2016;29:1038–49
Dorai T, Sawczuk IS, Pastorek J, Wiernik PH, Dutcher JP. The role of carbonic anhydrase IX overexpression in kidney cancer. Eur J Cancer 2005;41:2935–47
Kang HJ, Kim IH, Sung CO, Shim JH, Yu E. Expression of carbonic anhydrase 9 is a novel prognostic marker in resectable hepatocellular carcinoma. Virchows Arch 2015;466:403–13
Nault JC, De Reynies A, Villanueva A, et al. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology 2013;145:176–87
Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200–7
Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 2006;243:229–35
Inayoshi J, Ichida T, Sugitani S, et al. Gross appearance of hepatocellular carcinoma reflects E-cadherin expression and risk of early recurrence after surgical treatment. J Gastroenterol Hepatol 2003;18:673–7
Zakhary NI, Khodeer SM, Shafik HE, Abdel Malak CA. Impact of PIVKA-II in diagnosis of hepatocellular carcinoma. J Adv Res 2013;4:539–46
Hong YM, Cho M, Yoon KT, et al. Risk factors of early recurrence after curative hepatectomy in hepatocellular carcinoma. Tumour Biol 2017;39:1010428317720863
Lau EY, Lo J, Cheng BY, et al. Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep 2016;15:1175–89
Giannoni E, Bianchini F, Masieri L, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res 2010;70:6945–656
Zhang XH, Jin X, Malladi S, et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 2013;154:1060–73
Parikh JG, Kulkarni A, Johns C. alpha-smooth muscle actin-positive fibroblasts correlate with poor survival in hepatocellular carcinoma. Oncol Lett 2014;7:573–5
Muramatsu S, Tanaka S, Mogushi K, et al. Visualization of stem cell features in human hepatocellular carcinoma reveals in vivo significance of tumor-host interaction and clinical course. Hepatology 2013;58:218–28
Nahm JH, Rhee H, Kim H, et al. Increased expression of stemness markers and altered tumor stroma in hepatocellular carcinoma under TACE-induced hypoxia: a biopsy and resection matched study. Oncotarget 2017;8:99359–71
Luo D, Wang Z, Wu J, Jiang C, Wu J. The role of hypoxia inducible factor-1 in hepatocellular carcinoma. Biomed Res Int 2014;2014:409272
Carnero A, Lleonart M. The hypoxic microenvironment: a determinant of cancer stem cell evolution. BioEssays 2016;38(Suppl 1):S65–74
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) funded by the Korean Government (MSIP) (nos. NRF-2017R1A2B4005871, NRF-2017M3A9B6061512, and NRF-2016M3A9D5A01952416).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hyungjin Rhee, Taek Chung, Jeong Eun Yoo, Ji Hae Nahm, Ha Young Woo, Gi Hong Choi, Dai Hoon Han, and Young Nyun Park declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional national research committee (Institutional Review Board of Severance Hospital, IRB number: 4-2019-0172) and as per the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rhee, H., Chung, T., Yoo, J.E. et al. Gross type of hepatocellular carcinoma reflects the tumor hypoxia, fibrosis, and stemness-related marker expression. Hepatol Int 14, 239–248 (2020). https://doi.org/10.1007/s12072-020-10012-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-020-10012-6