Abstract
Herein, we have explored the enantioselective Michael addition of various malonate esters to benzalacetophenone by successful utilization of chiral phase transfer catalysts derived from proline, mandelic acid and tartaric acid under mild phase transfer conditions. The obtained results signify that these chiral phase transfer catalysts are efficacious towards enantioselective Michael addition as the use of it resulted in good enantioselectivity and appreciable chemical yields.
Graphic Abstract
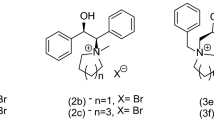
SYNOPSIS Highly enantioselective Michael addition of dialkyl malonates to benzalacetophenone has been established under mild phase transfer conditions, by successful utilization of chiral phase transfer catalysts derived from proline, mandelic acid and tartaric acid (I–VI) with appreciable chemical yields.





Similar content being viewed by others
References
(a) Li G Y, Zheng G and Noonan A F 2001 Highly active, air-stable versatile palladium catalysts for the C-C, C-N, and C-S bond formations via cross-coupling reactions of aryl chlorides J. Org. Chem. 66 8677; (b) Ritleng V, Sirlin C and Pfeffer M 2002 Ru-, Rh- and Pd-catalyzed C-C bond formation involving C-H activation and addition on unsaturated substrates: reactions and mechanistic aspects Chem. Rev. 102 1731; (c) Trost B M 1991 The atom economy—a search for synthetic efficiency Science. 254 1471; (d) Trost B M 1995 Atom economy-a challenge for organic synthesis: homogeneous catalysis leads the way Angew. Chem. Int. Ed. Engl. 34 259; (e) For a recent review, see: Tokoroyama T 2010 Eur. J. Org. Chem. 2009; (f) Rosini G In Comprehensive organic synthesis B M Trost, I Fleming and C H Heathcock (Eds.) 1991 Vol. 2 (Oxford: Pergamon) p. 321
(a) Sulzer M and Alexakis A 2007 Chiral amines as organocatalysts for asymmetric conjugate addition to nitroolefins and vinyl sulfones via enamine activation Chem. Commun. 38 3123; (b) Ballini R, Bosica G, Fiorini D, Palmieri A and Petrini M 2005 Conjugate additions of nitroalkanes to electron-poor alkenes: recent results Chem. Rev. 105 933
(a) Miyazaki T, Maekawa H, Yonemura K, Yamamoto Y, Yamanaka Y and Nishiguchi I 2011 Mg-promoted facile and selective intramolecular cyclization of aromatic \({\updelta }\)-ketoesters Tetrahedron 67; (b) Somaiah S, Sashikanth S, Raju V and Reddy K V 2011 An efficient and stereoselective synthesis of (\(3S\),\(4R\))-(-)-trans-4-(\(4^{\prime }\)-fluorophenyl)-3-hydroxymethyl-\(N\)-methylpiperidine Tetrahedron Asymm. 22 1
(a) Jacobsen E N, Pfaltz A and Yamamoto H (Eds.) 1999 In Comprehensive asymmetric catalysis; 1st ed., (Berlin: Springer); (b) Almasüi D, Alonso D A and Na’jera C 2007 Organocatalytic asymmetric conjugate additions. Tetrahedron: Asymm. 18 299; (c) Tsogoeva S B 2007 Recent advances in asymmetric organocatalytic 1,4- conjugate additions. Eur. J. Org. Chem. 11 1701; (d) Hayashi T and Yamasaki K 2003 Rhodium-catalyzed asymmetric 1,4-addition and its related asymmetric reactions Chem. Rev. 103 2829; (e) Christoffers J and Baro A 2003 Construction of quaternary stereocenters: new perspectives through enantioselective Michael reactions Angew. Chem. Int. Ed. 42 1688; (f) Berner O M, Tedeschi L and Enders D 2002 Asymmetric Michael additions to nitroalkenes Eur. J. Org. Chem. 12 1877; (g) Sibi M P and Manyem S 2000 Enantioselective conjugate additions Tetrahedron 56 8033; (h) Lippur K, Kaabel S, Järving I, Rissanen K and Kanger T 2015 \(\text{CaCl}_{2}\), Bisoxazoline, and malonate: a protocol for an asymmetric Michael reaction J. Org. Chem. 80 6336; (i) Naka H, Kanase N, Ueno M and Kondo Y 2008 Chiral bisphosphazides as dual basic enantioselective catalysts Chem. Eur. J. 14 5267
Wang Z, Wang Q, Zhang Y and Bao W 2005 Synthesis of new chiral ionic liquids from natural acids and their applications in enantioselective Michael addition Tetrahedron Lett. 46 4657
(a) Park S Y, Morimoto H, Matsunaga S and Shibasaki M 2007 Catalytic asymmetric Michael reactions of dibenzyl malonate to \({\upalpha }\), \({\upbeta }\)-unsaturated N-acylpyrroles using a La(O-iPr)3/Ph-linked-BINOL complex Tetrahedron Lett. 48 2815; (b) Chen C, Zhu S F, Wu X Y and Zhou Q L 2006 Preparation and application of chiral spiro nitrogen-containing ligands for cobalt-catalyzed asymmetric Michael addition Tetrahedron Asymm. 17 2761; (c) Velmathi S, Swarnalakshmi and Narasimhan S 2003 Heterobimetallic catalysts for asymmetric Michael reactions Tetrahedron: Asymm. 14 113; (d) Xu Y, Ohori K, Ohshima T and Shibasaki M A 2002 Practical large-scale synthesis of enantiomerically pure 3-[bis(methoxycarbonyl)methyl] cyclohexanone via catalytic asymmetric Michael reaction Tetrahedron 58 2585; (e) Kumaraswamy G, Sastry M N V and Jena N 2001 Calcium-BINOL: a and efficient catalyst for asymmetric Michael reactions Tetrahedron Lett. 42 8515; (f) Sasai H, Arai T, Satow Y, Houk K N and Shibasaki M 1995 The first heterobimetallic multifunctional asymmetric catalyst J. Am. Chem. Soc. 117 6194; (g) Ray S K, Singh P K and Singh V K 2011 Enantioselective michael addition of malonates to 2-enoylpyridine N-oxides catalyzed by chiral bisoxazoline\_Zn(II) Complex Org. Lett. 13 5812; (h) Espinosa M, Blay G, Cardona L and Pedro J R 2013 Asymmetric conjugate addition of malonate esters to a,b-unsaturated NSulfonyl imines: an expeditious route to chiral d-aminoesters and piperidones Chem. Eur. J. 19 14861
(a) Ooi T, Ohara D, Fukumoto K and Maruoka K 2005 Importance of chiral phase-transfer catalysts with dual functions in obtaining high enantioselectivity in the michael reaction of malonates and chalcone derivatives Org. Lett. 7 3195; (b) Dere R T, Pal R R, Patil P S and Salunkhe M M 2003 Influence of ionic liquids on the phase transfer-catalysed enantioselective Michael reaction Tetrahedron Lett. 44 5351; (c) Kim D Y, Huh S C and Kim S M 2001 Enantioselective Michael reaction of malonates and chalcones by phase-transfer catalysis using chiral quaternary ammonium salt Tetrahedron Lett. 42 6299
(a) Shioiri T, Sasson Y and Neumann R 1997 In Handbook of phase-transfer catalysis Sasson Y and Neumann R (Eds.) (London: Blackie Academic & Professional) Ch. 14; (b) Shioiri T and Arai S 2000 In Stimulating concepts in chemistry F Vogtle, J F Stoddart and M Shibasaki (Eds.) (Weinheim: Wiley-VCH) p. 123; (c) M J O’Donnell and I Ojima (Eds.) 2000 In Catalytic asymmetric syntheses \(2^{{\rm nd}}\) edn. (New York: Wiley-VCH) Ch. 10; (d) Maruoka K and Ooi T 2003 Enantioselective amino acid synthesis by chiral phase-transfer catalysis Chem. Rev. 103 3013; (e) O’Donnell M J 2004 The enantioselective synthesis of \({\upalpha }\)-amino acids by phase-transfer catalysis with achiral schiff base esters Acc. Chem. Res. 37 506; (f) Lygo B and Andrews B I 2004 Asymmetric phase-transfer catalysis utilizing chiral quaternary ammonium salts: asymmetric alkylation of glycine imines Acc. Chem. Res. 37 518; (g) Palvolgyi A, Rapi Z, Ozohanics O, Toth G, Keglevich G and Bako P 2018 Synthesis of alkyl \({\upalpha }\)-and \({\upbeta }\)-d-glucopyranoside-based chiral crown ethers and their application as enantioselective phase-transfer catalysts Res. Chem. Intermed. 44 1627; (h) Guo Wengang, Liu Xianghui, Liu Yan and Li Can 2018 Chiral catalysis at the water/oil interface ACS Catal. 8 328; (i) Woo S, Yong-Gyun K, Baegeun L, Jiin O, Yeonji L, Hyeri G and Keepyung N 2018 Dimeric cinchona ammonium salts with benzophenone linkers: enantioselective phase transfer catalysts for the synthesis of \({\upalpha }\)-amino acids RSC Adv. 8 2157; (j) Ha M W, Lee J Y, Kim D, Lee G, Lee J K, Hong S and Park H-G 2018 Enantioselective Synthesis of Chiral \(\alpha \)-Thio-Quaternary Stereogenic Centers via Phase-Transfer-Catalyzed \(\alpha \)-Alkylation of \(\alpha \)-Acylthiomalonates J. Org. Chem. 83 1011; (k) Nemcsok T, Rapi Z, Keglevich G and Bako G A 2018 Synthesis of d- mannitol- based crown ethers and their application as catalyst in asymmetric phase transfer reactions Chirality 30 407; (i) Shirakawa S and Maruoka K 2013 Recent developments in asymmetric phase-transfer reactions Angew. Chem. Int. Ed. 52 4312
Mahajan D P, Godbole H M, Singh G P and Shenoy G G 2019 Synthesis of phase transfer catalysts derived from proline-mandelic acid/tartaric acid: their evaluation in enantioselective epoxidation and Darzen condensation J. Chem. Sci. 131 22
Kosuke Y, Mitsuru S and Kenji K 2018 Enantioselective Michael reaction of ketone lithium enolates using a chiral amine ligand Tetrahedron Lett. 37 6343
Dongdong C, Guosheng F, Jiaxing Z, Hongyu W, Changwu Z and Gang Z 2016 Enantioselective Michael addition of malonates to chalcone derivatives catalyzed by dipeptide-derived multifunctional phosphonium salts J. Org. Chem. 81 9973
(a) The reported specific optical rotation for dimethyl (S)-2-(3-oxo-1,3-diphenylpropyl) malonate (3a) is \(21.0^{\circ }\) for 99% ee, \([{\upalpha }]_{{\rm D}}^{26.9}\) (\(c\) 1.0 in \(\text{ CHCl }_{3})^{11}\). So, the known SOR \([{\upalpha }]_{\uplambda }\) for 100% ee would be, \([21.0 \div 99{\rm x}100] = 21.212^{\circ }\). Optical purity (% ee) has been calculated by using formula. (b) F A Carey and R J Sundberg 2007 In A handbook of advanced organic chemistry, part A: structure and mechanisms 5\(^{th}\) edn. (Berlin: Springer)
The reported specific optical rotation for diethyl (S)-2-(3-oxo-1,3-diphenylpropyl) malonate (3b) is \([{\upalpha }]_{{\rm D}}^{28}= +18.5^{\circ }\) for 99% ee (\(c\) 1.0 in \(\text{ CHCl }_{3})^{11}\). So, the known SOR \([{\upalpha }]_{\uplambda }\) for 100% ee would be, \([18.5 \div 99{\rm x}100] = 18.687^{\circ }\). Optical purity (% ee) has been calculated by using formula. 12b
Kobayashi S, Agostinho M, Schneider U and Yamaguchi M 2011Catalysts and reaction process U. S. Patent US2011/54190 A1
Qinqin Q, Wenguo Z, Chengrong L, Bei Z and Yingming Y 2016 An efficient and practical enantiospecific synthesis of methyl chromanone- and chroman-2-carboxylates Tetrahedron Asymm. 27 911
The reported specific optical rotation for diisopropyl (S)-2-(3-oxo-1,3-diphenylpropyl) malonate (3c) is \([{\upalpha }]_{{\rm D}}^{28}18.9^{\circ }\) for 90% ee (\(c\) 0.925 in \(\text{ CHCl }_{3}\)).\(^{15}\) So, the known SOR \([{\upalpha }]_{\uplambda }\) for 100% ee would be, \([18.9 \div 90 {\rm x} 100] = 21.0^{\circ }\). Optical purity (% ee) has been calculated by using formula. 12b
Donghui C, Zhenling C, Xiao X, Zhigang Y, Lili L, Xiaohua L and Xiaoming F 2009 Highly Enantioselective Michael Addition of Malonates to Enones by N, N’-Dioxide Scandium(III) Complex Chem. Eur. J. 15 6807
The reported specific optical rotation for di-tert-butyl (S)-2-(3-oxo-1,3-diphenylpropyl) malonate (3d) is \([\upalpha ]_{{\rm D}}^{20} 23.4^{\circ }\) for 98% ee (\(c \) 0.24 in \(\text{ CHCl }_{3}\))\(^{17}\). So, the known SOR \([{\upalpha }]_{\uplambda }\) for 100% ee would be, \([23.4 \div 98 {\rm x}100] = 23.877^{\circ }\). Optical purity (% ee) has been calculated by using formula. [12b]
The reported specific optical rotation for dibenzyl (S)-2-(3-oxo-1,3-diphenylpropyl)malonat (3e) is \(12.5^{\circ }\) for 99% ee, \([{\upalpha }]_{{\rm D}}^{27.9}\) (\(c\) 0.97 in \(\text{ CHCl }_{3}\))\(^{11}\). So, the known SOR \([{\upalpha }]_{\uplambda }\) for 100% ee would be, \([12.5 \div 99{\rm x}100] = 12.626^{\circ }\). Optical purity (% ee) has been calculated by using formula. [12b]
Agostinho M and Kobayashi S 2008 Strontium-catalyzed highly enantioselective michael additions of malonates to enones J. Am. Chem. Soc. 130 2430
Wang J, Li H, Zu L, Jiang W, Xie H, Duan W and Wang W 2006 Organocatalytic enantioselective conjugate additions to enones J. Am. Chem. Soc. 128 12652
Acknowledgements
The authors are thankful to the M/S Lupin Limited and the authorities of Manipal Academy of Higher Education for the research program. We also acknowledge the valuable guidance, support and suggestions from Dr. P. R. Upadhaya and Dr. Vijaya Desai.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahajan, D.P., Godbole, H.M., Singh, G.P. et al. Enantioselective Michael addition of malonic esters to benzalacetophenone by using chiral phase transfer catalysts derived from proline-mandelic acid/tartaric acid. J Chem Sci 131, 67 (2019). https://doi.org/10.1007/s12039-019-1642-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-019-1642-5