Abstract
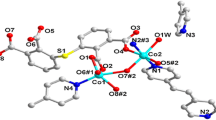
A hybrid compound (H2bbi)[Cu2(pzta)2(H2O)2][ β-Mo8O26] (1) (pztaH = 5-(2-pyrazinyl) tetrazolate, bbi = 1,1’-(1,4-butanediyl)bis(imidazole), has been hydrothermally synthesized and characterized by elemental analysis, IR spectroscopy and single-crystal X-ray diffraction. In 1, the β-Mo8 clusters link dinuclear copper (II) complexes as bidentate connectors to form inorganic-organic chains. These chains and the [H2bbi]2+ counter-cations are fused together forming layers via hydrogen bonding interactions. The electrochemical properties of 1 were studied. The results indicate that 1 has an electrocatalytic activity towards the reduction of iodate ascribed to the Mo-centers.

A new hybrid compound based on dinuclear copper complex has been synthesized, and its electrochemical properties were studied, which indicate that it has a good electrocatalytic activity toward reduction of iodate.






Similar content being viewed by others
References
Coronado E and Gómez-García C J 1998 Chem. Rev. 98 273
Müller A and Kögerler P 2000 Coord. Chem. Rev. 199 335
Han Q X, He C, Zhao M, Qi B, Niu J Y and Duan C Y 2013 J. Am. Chem. Soc. 135 10186
Yin Q S, Tan J M, Besson C, Geletii Y V, Musaev D G, Kuznetsov A E, Luo Z, Hardcastle K I and Hill C L 2010 Science 328 342
Hill C L 1998 Chem. Rev. 98 1
Han X B, Li Y G, Zhang Z M, Tan H Q, Lu Y and Wang E B 2015 J. Am. Chem. Soc. 137 5486
Yaghi O M, Keeffe M O, Ockwig N W, Chae H K, Eddaoudi M and Kim J 2003 Nature 423 705
Rowsell J L, Spencer E C, Eckert J, Howard J A and Yaghi O M 2005 Science 309 1350
Custelcean R 2014 Chem. Soc. Rev. 43 1813
Wang S S and Yang G Y 2015 Chem. Rev. 115 4892
Vaddypally S and Samar K D 2005 Inorg. Chem. 44 8846
Monima S, Tanmay C and Samar K D 2011 Dalton Trans. 40 2954
Du D Y, Yan L K, Su Z M, Li S L, Lan Y Q and Wang E B 2013 Coord. Chem. Rev. 257 702
Reinoso S, Vitoria P, Lezama L, Luque A and Gutiérrez-Zorrilla J M 2003 Inorg. Chem. 42 3709
Reinoso S, Vitoria P, Gutiérrez-Zorrilla J M, Lezama L S, Felices L and Beitia J I 2005 Inorg. Chem. 44 9731
Cao R G, Liu S X, Xie L H, Pan Y B, Cao J F, Ren Y H and Xu L 2007 Inorg. Chem. 46 3541
Yu F, Kong X J, Zheng Y Y, Ren Y P, Long L S, Huang R B and Zheng L S 2009 Dalton Trans. 9503
Han Q X, Ma P T, Zhao J W, Wang J P and Niu J Y 2011 Inorg. Chem. Commun. 14 767
Li S B, Ma H Y, Pang H J, Zhang Z F, Yu Y, Liu H and Yu T T 2014 Cryst. Eng. Comm. 16 2045
Li S B, Ma H Y, Pang H J, Li Z and Zhang Z F 2014 Inorg. Chem. Commun. 44 15
Sheldrick GM (2000) shelxtl (version 6.1) (Bruker Analytical, X-ray Instruments Inc. : Madison, Wisconsin)
Li S L, Lan Y Q, Ma J F, Yang J, Wang X H and Su Z M 2007 Inorg. Chem. 46 8283
Allis D G, Burkholder E and Zubieta J 2004 Polyhedron 23 1145
Wu H, Yang J, Liu Y Y and Ma J F 2012 Cryst. Growth Des. 12 2272
Lan Y Q, Li S L, Wang X L, Shao K Z, Su Z M and Wang E B 2008 Inorg. Chem. 47 529
Brown I D and Altermatt D 1985 Acta Crystallogr. B 41 244
Sha J Q, Peng J, Zhang Y, Pang H J, Tian A X, Zhang P P and Liu H 2009 Cryst. Growth Des. 9 1708
Klemperer W G and Shum W 1976 J. Am. Chem. Soc. 98 8291
Xi X D, Wang G, Liu B F and Dong S J 1995 Electrochim. Acta 40 1025
Han Z G, Zhao Y L, Peng J, Feng Y H, Yin J N and Liu Q 2005 Electroanalysis 17 1097
Dong S J and Wang B X 1992 Electrochim. Acta 37 11
Dai L M, You W S, Wang E B, Wu S X, Su Z M, Du Q H, Zhao Y and Fang Y 2009 Cryst. Growth Des. 92 110
Sha J Q, Liang L Y, Sun J W, Tian A X, Yan P F, Li G M and Wang C 2012 Cryst. Growth Des. 12 894
Keita B, Oliveira P D, Nadjo L and Kortz U 2007 Chem. Eur. J. 13 5480
Qu Z K, Yu K, Zhao Z F, Su Z H, Sha J Q, Wang C M and Zhou B B 2014 Dalton Trans. 43 6744
Qin Q S, Du D Y, Guan W, Bo X J, Li Y F, Guo L P, Su Z M, Wang Y Y, Lan Y Q and Zhou H C 2015 J. Am. Chem. Soc. 137 7169
Keita B, Belhouari A, Nadjo L and Contant R 1995 J. Electroanal. Chem. 381 243
Acknowledgements
This work was financially supported by the NSF of China (21371041, 51572063), innovative research team of green chemical technology in university of Heilongjiang Province, China (2014TD007), and the science and technology innovation foundation of Harbin (2014RFXXJ076).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information (SI)
CIF file containing complete information on the structure was deposited with CCDC deposition number 992478, which is available free upon request from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The table of selected bond lengths and angles and the IR spectrum of compound 1 are given in supplementary information available at www.ias.ac.in/chemsci.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
LI, S., ZHANG, L., MA, H. et al. Hydrothermal synthesis and electrochemical properties of a coordination polymer based on dinuclear (Pyrazinyl tetrazolate) Copper(II) cations and β-Octamolybdate Anions. J Chem Sci 128, 825–830 (2016). https://doi.org/10.1007/s12039-016-1076-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1076-2