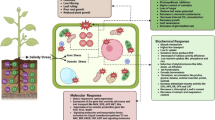
Abstract
We correlated root growth inhibition with aluminium (Al3+) localization and toxicity symptoms in rice roots using seedlings of two genotypes (tolerant and sensitive) that were exposed to different AlCl3 concentrations. Al3+ localization was evaluated by hematoxylin in primary roots and by morin in cross-sections of the root tips. Neutral invertase enzyme activity and callose (1→3, β-d-glucan) accumulation were observed and compared with Al3+ accumulation sites. Root growth was inhibited by Al3+ in a concentration-specific manner and proportional to the increase of hematoxylin staining, being more pronounced in the sensitive genotype. Morin staining showed the presence of Al3+ deep within the roots of the sensitive genotype, indicating that the metal was able to penetrate beyond the first few cell layers. In the tolerant genotype, Al3+ penetration was restricted to the first two cell layers. Ruptures in exodermis and epidermis layers by lateral root protrusions in both genotypes allowed Al3+ to enter into the roots. More intense activity of invertase in roots of the tolerant genotype was also observed, which could be related to greater root growth of this cultivar when submitted to Al3+ stress. Moreover, Al3+-induced callose accumulation was a late response occurring in the same areas where Al3+ was present.






Similar content being viewed by others
References
Alvarez I, Sam O, Reynaldo I, Testillano P, Risueno MC and Arias M 2012 Morphological and cellular changes in rice roots (Oryza sativa L.) caused by Al stress. Bot. Studies 53 67–73
Baker JR 1962 Experiments on the action of mordants. 2. Aluminuim-haematein. Quart. J. Micr. Sci. 103 493–517
Bhuja P, McLachlan K, Stephens J and Taylor G 2004 Accumulation of 1,3-beta-D-glucans, in response to aluminum and cytosolic calcium in Triticum aestivum. Plant Cell Physiol. 45 543–549
Cai M, Zhang S, Xing C, Wang F and Lei Zhu N 2011 Developmental characteristics and aluminum resistance of root border cells in rice seedlings. Plant Sci. 180 702–708
Degenhardt J, Larsen PB, Howell SH and Kochian LV 1998 Aluminum resistance in the Arabdopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiol. 117 19–27
Delhaize E and Ryan PR 1995 Aluminum toxicity and tolerance in plants. Plant Physiol. 107 315–321
Doehlert DC and Felker FC 1987 Characterization and distribution of invertase activity in developing maize (Zea mays) kernels. Physiol. Plant. 70 51–57
Eggert DA 1970 Use of morin for fluorescent localization of aluminum in plant tissues. Stain Tech. 45 301–303
Eticha D, Stass A and Horst WJ 2005 Localization of aluminium in the maize root apex: can morin detect cell wall-bound aluminium? J. Exp. Bot. 56 1351–1357
Ezaki B, Gardner RC, Ezaki Y and Matsumoto H 2000 Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/ or oxidative stress. Plant Physiol. 122 657–665
Foy CD, Chaney RL and White MC 1978 Physiology of metal toxicity in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 29 511–566
Horst WJ, Wang Y and Eticha D 2010 The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann. Bot. 106 185–197
Kauss H 1992 Callose and callose synthase; in Molecular plant morphology: A practical approach (eds) SJ Gurr, MJ McPherson and DJ Bowles (Oxford University Press) pp 1–8
Koch K 2004 Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Op. Plant Biol. 7 235–246
Kochian LV 1995 Cellular mechanisms of aluminum toxicity and resistance in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 46 237–260
Larsen PB, Tai CY, Kochian LV and Howell SH 1996 Arabidopsis mutants with increased sensitivity to aluminum. Plant Physiol. 110 743–751
Lazof DB, Goldsmith JG, Rufty TW and Linton RW 1996 The early entry of Al into cells of intact soybean roots – A comparison of three developmental root region using secondary ion mass spectrometry imaging. Plant Physiol. 112 1289–1300
Li XF, Ma JF, Hiradate S and Matsumoto H 2000 Mucilage strongly binds aluminum but does not prevent roots from aluminum injury in Zea mays. Physiol. Plant. 108 152–160
Ma JF 2000 Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol. 41 383–390
Ma JF, Shen RF, Zhao ZQ, Wissuwa M, Takeuchi Y, Ebitani T and Yano M 2002 Response of rice to Al stress and identification of quantitative trait loci for Al tolerance. Plant Cell Physiol. 43 652–659
Marciano DPRO, Ramos FT, Alvim MN, Magalhaes JR and França MGC 2010 Nitric oxide reduces the stress effects of aluminum on the process of germination and early root growth of rice. J. Plant Nut. Soil Sci. 173 885–891
Matsumoto H, Senoo Y, Kasai M and Maeshima M 1996 Response of the plant root to aluminum stress: Analysis of the inhibition of the root elongation and changes in membrane function. J. Plant Res. 109 99–105
Nagy NE, Dalen LS, Jones DL, Swensen B, Fossdal CG and Eldhuset TD 2004 Cytological and enzimatic responses to aluminium stress in root tips of Norway spruce seedlings. New Phytol. 163 595–607
Ramos FT, Rossiello ROP, França MGC, Alvim MN and Olivares FL 2007 Aluminum hematoxylin complex indicates the mucilage contribution to Al tolerance in root apex of rice. Physiol. Mol. Bio. Plants 13 9–16
Rincon M and Gonzales RA 1992 Aluminum partioning in intact root of aluminum-tolerant and aluminum-sensitive wheat (Triticum aestivum L.) cultivars. Plant Physiol. 99 1021–1028
Samac DA and Tesfaye M 2003 Plant improvement for tolerance to aluminum in acid soils – a review. Plant Cell Tissue Organ Cult. 75 189–207
Silva IR, Smyth TJ, Raper CD, Carter TE and Rufty TW 2001 Differential aluminum tolerance in soybean: An evaluation of the role of organic acids. Physiol. Plant. 112 200–210
Simon L, Kieger M, Sung SS and Smalley TJ 1994 Aluminum toxicity in tomato. Part 2. Leaf gas exchange, chlorophyll content, and invertase activity. J. Plant Nut. 17 307–317
Sivaguru M, Fujiwara T, Samaj J, Baluska F, Yang ZM, Osawa H, Maeda T, Mori T, Volkmann D and Matsumoto H 2000 Aluminum-induced 1→3, β-D-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiol. 124 991–1005
Sivaguru M and Horst WJ 1998 The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiol. 116 155–163
Taylor GJ, Basu A, Basu U, Slaski JJ, Zhang GC and Good A 1997 Al-induced, 51-kilodalton, membrane-bound proteins are associated with resistance to Al in a segregating population of wheat. Plant Physiol. 114 363–372
Tice KR, Parker DR and Demason DA 1992 Operationally defined apoplastic and symplastic aluminum fractions in root tips of aluminum-intoxicated wheat. Plant Physiol. 100 309–318
Verma DPS and Hong Z 2001 Plant callose synthase complexes. Plant Mol. Biol. 47 693–701
Yamamoto Y, Kobayashi Y and Matsumoto H 2001 Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 125 199–208
Yang JL, Li YY, Zhang YJ, Wu YR, Wu P and Zheng SJ 2008 Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol. 146 602–611
Zhang GC, Hoddinott J and Taylor GJ 1994 Characterization of 1,3-beta-D-Glucan (Callose) synthesis in roots of Triticum aestivum in response to aluminum toxicity. J. Plant Physiol. 144 229–234
Zrenner R, Salanoubat M, Willmitzer L and Sonnnewald U 1995 Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J. 7 97–107
Acknowledgements
We are grateful to Plant Anatomy and Mycology Laboratories for analytical support of this work. Our work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We are grateful to Alistair Hayward for his critical review of the English text.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Renu Khanna-Chopra
[Alvim MN, Ramos FT, Oliveira DC, Isaias RMS and França MGC 2012 Aluminium localization and toxicity symptoms related to root growth inhibition in rice (Oryza sativa L.) seedlings. J. Biosci. 37 1–10] DOI 10.1007/s12038-012-9275-6
Rights and permissions
About this article
Cite this article
Alvim, M.N., Ramos, F.T., Oliveira, D.C. et al. Aluminium localization and toxicity symptoms related to root growth inhibition in rice (Oryza sativa L.) seedlings. J Biosci 37 (Suppl 1), 1079–1088 (2012). https://doi.org/10.1007/s12038-012-9275-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-012-9275-6