Abstract
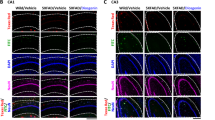
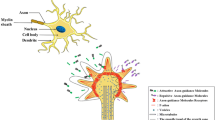
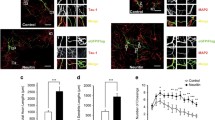
Galectin-1 (Gal-1), a member of the Galectin family, is expressed in various tissues and responsible for multiple biological activities. Previous studies reported that extracellular Gal-1 participated in axonal growth and repair, and Gal-1 knockout mice exhibited memory impairment. However, no study has demonstrated the direct contribution of intracellular Gal-1 upregulation in neurons to promoting axonal regeneration in the brain and recovering memory function. In the present study, we found that axonal growth is promoted by overexpression of Gal-1 via adeno-associated virus serotype 9 delivery in primary cultured hippocampal neurons. Moreover, Gal-1 was expressed on the membranes of growth cones in hippocampal neurons and interacted with a novel axonal guidance molecule, Secernin-1, which was secreted from prefrontal cortex (PFC) neurons. Gal-1-overexpression-driven axonal growth was enhanced when recombinant (extracellular) Secernin-1 was treated to the axonal site in a neuron device chamber. Direct binding of extracellular Secernin-1 with Gal-1 was detected through immunoprecipitation and immunocytochemistry, demonstrating that Gal-1 possibly works as an axonal guidance receptor for Secernin-1 in hippocampal neurons. In the PFC, the expression of Gal-1 in axonal shafts and terminals of hippocampal neurons was decreased in the 5XFAD mouse model of Alzheimer’s disease (AD). Overexpression of Gal-1 in hippocampal neurons recovered memory deficits and induced axonal regeneration toward the PFC in 5XFAD mice. This study suggests that the enhanced interaction of Secernin-1 and Gal-1 can be harnessed as a therapeutic strategy for long-distance and direction-specific axonal regeneration in AD.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Camby I, Le Mercier M, Lefranc F, Kiss R (2006) Galectin-1: a small protein with major functions. Glycobiology 16:137R-157R. https://doi.org/10.1093/glycob/cwl025
Horie H, Inagaki Y, Sohma Y, Nozawa R, Okawa K, Hasegawa M et al (1999) Galectin-1 regulates initial axonal growth in peripheral nerves after axotomy. J Neurosci 19:9964–9974. https://doi.org/10.1523/JNEUROSCI.19-22-09964.1999
McGraw J, McPhail LT, Oschipok LW, Horie H, Poirier F, Steeves JD et al (2004) Galectin-1 in regenerating motorneurons. Eur J Neurosci 20:2872–2880. https://doi.org/10.1111/j.1460-9568.2004.03802.x
Horie H, Kadoya T, Hikawa N, Sango K, Inoue H, Takeshita K et al (2004) Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. J Neurosci 24:1873–1880. https://doi.org/10.1523/JNEUROSCI.4483-03.2004
Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T et al (1994) Galectins: a family of animal beta-galactoside-binding lectins. Cell 76:597–598. https://doi.org/10.1016/0092-8674(94)90498-7
Mahanthappa NK, Cooper DN, Barondes SH, Schwarting GA (1994) Rat olfactory neurons can utilize the endogenous lectin, L-14, in a novel adhesion mechanism. Development 120:1373–1384. https://doi.org/10.1242/dev.120.6.1373
Puche AC, Poirier F, Hai M, Bartlett PF, Key B (1996) Role of galectin-1 in the developing mouse olfactory system. Dev Biol 179:274–287. https://doi.org/10.1006/dbio.1996.0257
Tenne-Brown J, Puche AC, Key B (1998) Expression of galectin-1 in the mouse olfactory system. Int J Dev Biol 42:791–799
Quintá HR, Pasquini JM, Rabinovich GA, Pasquini LA (2014) Glycan-dependent binding of galectin-1 to neuropilin-1 promotes axonal regeneration after spinal cord injury. Cell Death Differ 21:941–955. https://doi.org/10.1038/cdd.2014.14
Gaudet AD, Sweet DR, Polinski NK, Guan Z, Popovich PG (2015) Galectin-1 in injured rat spinal cord: implications for macrophage phagocytosis and neural repair. Mol Cell Neurosci 64:84–94. https://doi.org/10.1016/j.mcn.2014.12.006
Wu G, Lu ZH, André S, Gabius HJ, Ledeen RW (2016) Functional interplay between ganglioside GM1 and cross-linking galectin-1 induces axon-like neuritogenesis via integrin-based signaling and TRPC5-dependent Ca2+ influx. J Neurochem 136:550–563. https://doi.org/10.1111/jnc.13418
Kajitani K, Kobayakawa Y, Nomaru H, Kadoya T, Horie H, Nakabeppu Y (2014) Characterization of galectin-1-positive cells in the mouse hippocampus. NeuroReport 25:171–176. https://doi.org/10.1097/WNR.0000000000000068
Quintá HR, Wilson C, Blidner AG, González-Billault C, Pasquini LA, Rabinovich GA et al (2016) Ligand-mediated galectin-1 endocytosis prevents intraneural H2O2 production promoting F-actin dynamics reactivation and axonal re-growth. Exp Neurol 283:165–178. https://doi.org/10.1016/j.expneurol.2016.06.009
Sakaguchi M, Arruda-Carvalho M, Kang NH, Imaizumi Y, Poirier F, Okano H et al (2011) Impaired spatial and contextual memory formation in galectin-1 deficient mice. Mol Brain 4:33. https://doi.org/10.1186/1756-6606-4-33
Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J et al (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci 26:10129–10140. https://doi.org/10.1523/JNEUROSCI.1202-06.2006
Tohda C, Urano T, Umezaki M, Nemere I, Kuboyama T (2012) Diosgenin is an exogenous activator of 1,25D3-MARRS/Pdia3/ERp57 and improves Alzheimer’s disease pathologies in 5XFAD mice. Sci Rep 2:535. https://doi.org/10.1038/srep00535
Yang X, Nomoto K, Tohda C (2021) Diosgenin content is a novel criterion to assess memory enhancement effect of yam extracts. J Nat Med 75:207–216. https://doi.org/10.1007/s11418-020-01451-4
Yang X, Tohda C (2018) Diosgenin restores Aβ-induced axonal degeneration by reducing the expression of heat shock cognate 70 (HSC70). Sci Rep 8:11707. https://doi.org/10.1038/s41598-018-30102-8
Yang X, Tohda C (2018) Heat shock cognate 70 inhibitor, VER-155008, reduces memory deficits and axonal degeneration in a mouse model of Alzheimer’s disease. Front Pharmacol 9:48.https://doi.org/10.3389/fphar.2018.00048
Bojic-Trbojevic Ž, Jovanovic Krivokuca M, Stefanoska I, Kolundžic N, Vilotic A, Kadoya T et al (2018) Integrin β1 is bound to galectin-1 in human trophoblast. J Biochem 163:39–50. https://doi.org/10.1093/jb/mvx061
Wang C, Furlong TM, Stratton PG, Lee C, Xu L, Merlin S et al (2021) Hippocampus-prefrontal coupling regulates recognition memory for novelty discrimination. J Neurosci 41:9617–9632. https://doi.org/10.1523/JNEUROSCI.1202-21.2021
Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S et al (2014) A mesoscale connectome of the mouse brain. Nature 508:207–214. https://doi.org/10.1038/nature13186
Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL et al (2010) An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci 13:1496–1504. https://doi.org/10.1038/nn.2674
Way G, Morrice N, Smythe C, O’Sullivan AJ (2002) Purification and identification of Secernin, a novel cytosolic protein that regulates exocytosis in mast cells. Mol Biol Cell 13:3344–3354. https://doi.org/10.1091/mbc.e01-10-0094
Lindhout FW, Cao Y, Kevenaar JT, Bodzęta A, Stucchi R, Boumpoutsari MM et al (2019) VAP-SCRN1 interaction regulates dynamic endoplasmic reticulum remodeling and presynaptic function. EMBO J 38:e101345. https://doi.org/10.15252/embj.2018101345
Drummond E, Wisniewski T (2017) The use of localized proteomics to identify the drivers of Alzheimer’s disease pathogenesis. Neural Regen Res 12:912–913. https://doi.org/10.4103/1673-5374.208570
Pires G, McElligott S, Drusinsky S, Halliday G, Potier MC, Wisniewski T et al (2019) Secernin-1 is a novel phosphorylated tau binding protein that accumulates in Alzheimer’s disease and not in other tauopathies. Acta Neuropathol Commun 7:195. https://doi.org/10.1186/s40478-019-0848-6
He Z, Jin Y (2016) Intrinsic control of axon regeneration. Neuron 90(3):437–451
Blazquez-Llorca L, Valero-Freitag S, Rodrigues EF, Merchán-Pérez Á, Rodríguez JR, Dorostkar MM et al (2017) High plasticity of axonal pathology in Alzheimer’s disease mouse models. Acta Neuropathol Commun 5:14. https://doi.org/10.1186/s40478-017-0415-y
Jin Y, Dougherty SE, Wood K, Sun L, Cudmore RH, Abdalla A et al (2016) Regrowth of serotonin axons in the adult mouse brain following injury. Neuron 91:748–762. https://doi.org/10.1016/j.neuron.2016.07.024
Takaku S, Yanagisawa H, Watabe K, Horie H, Kadoya T, Sakumi K et al (2013) GDNF promotes neurite outgrowth and upregulates galectin-1 through the RET/PI3K signaling in cultured adult rat dorsal root ganglion neurons. Neurochem Int 62:330–339. https://doi.org/10.1016/j.neuint.2013.01.008
Delbrouck C, Doyen I, Belot N, Decaestecker C, Ghanooni R, de Lavareille A et al (2002) Galectin-1 is overexpressed in nasal polyps under budesonide and inhibits eosinophil migration. Lab Invest 82:147–158. https://doi.org/10.1038/labinvest.3780407
Choe YS, Shim C, Choi D, Lee CS, Lee KK, Kim K (1997) Expression of galectin-1 mRNA in the mouse uterus is under the control of ovarian steroids during blastocyst implantation. Mol Reprod Dev 48:261–266. https://doi.org/10.1002/(SICI)1098-2795(199710)48:2%3c261::AID-MRD14%3e3.0.CO;2-0
Lu Y, Lotan D, Lotan R (2000) Differential regulation of constitutive and retinoic acid-induced galectin-1 gene transcription in murine embryonal carcinoma and myoblastic cells. Biochim Biophys Acta 1491:13–19. https://doi.org/10.1016/s0167-4781(00)00055-5
Ohannesian DW, Lotan D, Lotan R (1994) Concomitant increases in galectin-1 and its glycoconjugate ligands (carcinoembryonic antigen, lamp-1, and lamp-2) in cultured human colon carcinoma cells by sodium butyrate. Cancer Res 54:5992–6000
Kultima K, Nyström AM, Scholz B, Gustafson AL, Dencker L, Stigson M (2004) Valproic acid teratogenicity: a toxicogenomics approach. Environ Health Perspect 112:1225–1235. https://doi.org/10.1289/txg.7034
Akazawa C, Nakamura Y, Sango K, Horie H, Kohsaka S (2004) Distribution of the galectin-1 mRNA in the rat nervous system: its transient upregulation in rat facial motor neurons after facial nerve axotomy. Neuroscience 125:171–178. https://doi.org/10.1016/j.neuroscience.2004.01.034
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing. Moreover, we would like to thank the support of the 2021 Director Leadership Expenses, Institute of Natural Medicine, University of Toyama.
Funding
This work was funded by JSPS KAKENHI, Grant Number 19K16288 (X. Y.), a grant for young researcher of Hokuriku Bank, LTD (X. Y.), a grant from the Research Center for Idling Brain Science (X. Y.), a foundation from Tamura Science Technology (X. Y.), Grant-in-Aid for a Cooperative Research Project from the Institute of Natural Medicine at the University of Toyama in 2017–2020 (C. T.), and the Discretionary Funds of the President of the University of Toyama (C. T.). The funding bodies were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Author information
Authors and Affiliations
Contributions
X. Y. and C. T. designed the experiments and drafted the manuscript. X. Y. performed the experiments and analyzed the data. X. Y. and C. T. acquired funding.
Corresponding author
Ethics declarations
Ethics Approval
All experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the University of Toyama. The Committee for Animal Care and Use at the Sugitani Campus of the University of Toyama approved the study protocol (approval number: A2020INM-1).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, X., Tohda, C. Axonal Regeneration Mediated by a Novel Axonal Guidance Pair, Galectin-1 and Secernin-1. Mol Neurobiol 60, 1250–1266 (2023). https://doi.org/10.1007/s12035-022-03125-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03125-6