Abstract
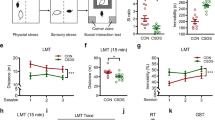
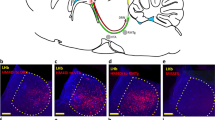
A common phenomenon called social buffering (SB), communication within conspecific animals is a benefit for a stressed individual to better recover from aversive events, is crucial to all mammals. Although the dopamine reward system has been implicated in SB, it is not clear which neuronal populations are relevant and how they contribute. Here, we adopted a learned helplessness (LH) animal model of depression and found that LH subjects housed with a conspecific partner show better performance in the shuttle box test, showing that SB improves the stress-coping abilities to deal with stress. Bidirectional manipulation of ventral tegmental area (VTA) dopamine neurons by chemogenetic tools can mimic or block the SB effect in LH mice. To screen for SB-induced structure plasticity of VTA dopamine neurons, we employed viral genetic tools for mapping input and output architecture and found LH- and SB-triggered circuit-level changes in neuronal ensembles. Zona incerta (ZI), an overlapping brain region, was significantly changed in both anterograde and retrograde tracing during LH and SB. These results reveal a neural loop with structural plasticity between VTA dopamine neurons and ZI underlies the SB effects in LH and lays a foundation for studying how VTA dopamine neurons regulate SB-related neural circuits.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Whitton AE, Treadway MT, Pizzagalli DA (2015) Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry 28(1):7–12. https://doi.org/10.1097/YCO.0000000000000122
Yang Y, Wang ZH, Jin S, Gao D, Liu N, Chen SP, Zhang S, Liu Q, Liu E, Wang X, Liang X, Wei P, Li X, Li Y, Yue C, Li HL, Wang YL, Wang Q, Ke D, Xie Q, Xu F, Wang L, Wang JZ (2016) Opposite monosynaptic scaling of BLP-vCA1 inputs governs hopefulness- and helplessness-modulated spatial learning and memory. Nat Commun 7:11935. https://doi.org/10.1038/ncomms11935
Nobrega JN, Hedayatmofidi PS, Lobo DS (2016) Strong interactions between learned helplessness and risky decision-making in a rat gambling model. Sci Rep 6:37304. https://doi.org/10.1038/srep37304
Slattery DA, Cryan JF (2017) Modelling depression in animals: at the interface of reward and stress pathways. Psychopharmacology 234(9–10):1451–1465. https://doi.org/10.1007/s00213-017-4552-6
Pittenger C, Duman RS (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33(1):88–109. https://doi.org/10.1038/sj.npp.1301574
Moreines JL, Owrutsky ZL, Grace AA (2017) Involvement of infralimbic prefrontal cortex but not lateral habenula in dopamine attenuation after chronic mild stress. Neuropsychopharmacology 42(4):904–913. https://doi.org/10.1038/npp.2016.249
Ries AS, Hermanns T, Poeck B, Strauss R (2017) Serotonin modulates a depression-like state in drosophila responsive to lithium treatment. Nat Commun 8:15738. https://doi.org/10.1038/ncomms15738
Muir J, Lorsch ZS, Ramakrishnan C, Deisseroth K, Nestler EJ, Calipari ES, Bagot RC (2018) In vivo fiber photometry reveals signature of future stress susceptibility in nucleus accumbens. Neuropsychopharmacology 43(2):255–263. https://doi.org/10.1038/npp.2017.122
Plotnik JM, de Waal FB (2014) Asian elephants (Elephas maximus) reassure others in distress. PeerJ 2:e278. https://doi.org/10.7717/peerj.278
Lei K, Liu Y, Smith AS, Lonstein JS, Wang Z (2017) Effects of pair bonding on parental behavior and dopamine activity in the nucleus accumbens in male prairie voles. Eur J Neurosci 46(7):2276–2284. https://doi.org/10.1111/ejn.13673
Kiyokawa Y, Ishida A, Takeuchi Y, Mori Y (2016) Sustained housing-type social buffering following social housing in male rats. Physiol Behav 158:85–89. https://doi.org/10.1016/j.physbeh.2016.02.040
Culbert BM, Gilmour KM, Balshine S (2019) Social buffering of stress in a group-living fish. Proc Biol Sci 286(1910):20191626. https://doi.org/10.1098/rspb.2019.1626
DeVries AC, Glasper ER, Detillion CE (2003) Social modulation of stress responses. Physiol Behav 79(3):399–407. https://doi.org/10.1016/s0031-9384(03)00152-5
Kikusui T, Winslow JT, Mori Y (2006) Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci 361(1476):2215–2228. https://doi.org/10.1098/rstb.2006.1941
Koolhaas JM, de Boer SF, Buwalda B, Meerlo P (2017) Social stress models in rodents: towards enhanced validity. Neurobiol Stress 6:104–112. https://doi.org/10.1016/j.ynstr.2016.09.003
Papantoni A, Reinblatt SP, Findling RL, Moran TH, Mogayzel PJ Jr, Carnell S (2019) Appetitive characteristics in children with cystic fibrosis: questionnaire validation and associations with nutritional status. Appetite 139:90–94. https://doi.org/10.1016/j.appet.2019.03.034
Kiyokawa Y, Honda A, Takeuchi Y, Mori Y (2014) A familiar conspecific is more effective than an unfamiliar conspecific for social buffering of conditioned fear responses in male rats. Behav Brain Res 267:189–193. https://doi.org/10.1016/j.bbr.2014.03.043
Nakamura K, Ishii A, Kiyokawa Y, Takeuchi Y, Mori Y (2016) The strain of an accompanying conspecific affects the efficacy of social buffering in male rats. Horm Behav 82:72–77. https://doi.org/10.1016/j.yhbeh.2016.05.003
Kiyokawa Y, Kawai K, Takeuchi Y (2018) The benefits of social buffering are maintained regardless of the stress level of the subject rat and enhanced by more conspecifics. Physiol Behav 194:177–183. https://doi.org/10.1016/j.physbeh.2018.05.027
Baron-Cohen S (2009) Autism: the empathizing-systemizing (E-S) theory. Ann N Y Acad Sci 1156:68–80. https://doi.org/10.1111/j.1749-6632.2009.04467.x
Dalla C, Edgecomb C, Whetstone AS, Shors TJ (2008) Females do not express learned helplessness like males do. Neuropsychopharmacology 33(7):1559–1569. https://doi.org/10.1038/sj.npp.1301533
Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M (2011) Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc Natl Acad Sci U S A 108(50):19937–19942. https://doi.org/10.1073/pnas.1113079108
Smith AS, Wang Z (2014) Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry 76(4):281–288. https://doi.org/10.1016/j.biopsych.2013.09.017
Sullivan RM, Perry RE (2015) Mechanisms and functional implications of social buffering in infants: lessons from animal models. Soc Neurosci 10(5):500–511. https://doi.org/10.1080/17470919.2015.1087425
Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ (2016) Oxytocin-dependent consolation behavior in rodents. Science 351(6271):375–378. https://doi.org/10.1126/science.aac4785
McQuaid RJ, McInnis OA, Paric A, Al-Yawer F, Matheson K, Anisman H (2016) Relations between plasma oxytocin and cortisol: the stress buffering role of social support. Neurobiol Stress 3:52–60. https://doi.org/10.1016/j.ynstr.2016.01.001
Doom JR, Doyle CM, Gunnar MR (2017) Social stress buffering by friends in childhood and adolescence: effects on HPA and oxytocin activity. Soc Neurosci 12(1):8–21. https://doi.org/10.1080/17470919.2016.1149095
Yadid G, Friedman A (2008) Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res 172:265–286. https://doi.org/10.1016/S0079-6123(08)00913-8
Morales M, Margolis EB (2017) Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 18(2):73–85. https://doi.org/10.1038/nrn.2016.165
Fernandez SP, Broussot L, Marti F, Contesse T, Mouska X, Soiza-Reilly M, Marie H, Faure P et al (2018) Mesopontine cholinergic inputs to midbrain dopamine neurons drive stress-induced depressive-like behaviors. Nat Commun 9(1):4449. https://doi.org/10.1038/s41467-018-06809-7
Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R (2011) Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 470(7335):535–539. https://doi.org/10.1038/nature09742
Bariselli S, Hornberg H, Prevost-Solie C, Musardo S, Hatstatt-Burkle L, Scheiffele P, Bellone C (2018) Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction. Nat Commun 9(1):3173. https://doi.org/10.1038/s41467-018-05382-3
Landgraf D, Long J, Der-Avakian A, Streets M, Welsh DK (2015) Dissociation of learned helplessness and fear conditioning in mice: a mouse model of depression. PLoS ONE 10(4):e0125892. https://doi.org/10.1371/journal.pone.0125892
Shin S, Kwon O, Kang JI, Kwon S, Oh S, Choi J, Kim CH, Kim DG (2015) mGluR5 in the nucleus accumbens is critical for promoting resilience to chronic stress. Nat Neurosci 18(7):1017–24. https://doi.org/10.1038/nn.4028
Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N (2012) Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74(5):858–873. https://doi.org/10.1016/j.neuron.2012.03.017
Beier KT, Kim CK, Hoerbelt P, Hung LW, Heifets BD, DeLoach KE, Mosca TJ, Neuner S et al (2017) Rabies screen reveals GPe control of cocaine-triggered plasticity. Nature 549(7672):345–350. https://doi.org/10.1038/nature23888
Kim JY, Yang SH, Kwon J, Lee HW, Kim H (2017) Mice subjected to uncontrollable electric shocks show depression-like behaviors irrespective of their state of helplessness. Behav Brain Res 322(Pt A):138–144. https://doi.org/10.1016/j.bbr.2017.01.008
Fraser ON, Stahl D, Aureli F (2008) Stress reduction through consolation in chimpanzees. Proc Natl Acad Sci U S A 105(25):8557–8562. https://doi.org/10.1073/pnas.0804141105
Faustino AI, Tacao-Monteiro A, Oliveira RF (2017) Mechanisms of social buffering of fear in zebrafish. Sci Rep 7:44329. https://doi.org/10.1038/srep44329
Kiyokawa Y, Takeuchi Y, Nishihara M, Mori Y (2009) Main olfactory system mediates social buffering of conditioned fear responses in male rats. Eur J Neurosci 29(4):777–785. https://doi.org/10.1111/j.1460-9568.2009.06618.x
Mulej Bratec S, Xie X, Schmid G, Doll A, Schilbach L, Zimmer C, Wohlschlager A, Riedl V et al (2015) Cognitive emotion regulation enhances aversive prediction error activity while reducing emotional responses. Neuroimage 123:138–148. https://doi.org/10.1016/j.neuroimage.2015.08.038
Takahashi Y, Kiyokawa Y, Kodama Y, Arata S, Takeuchi Y, Mori Y (2013) Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behav Brain Res 240:46–51. https://doi.org/10.1016/j.bbr.2012.11.017
Sanchez MM, McCormack KM, Howell BR (2015) Social buffering of stress responses in nonhuman primates: maternal regulation of the development of emotional regulatory brain circuits. Soc Neurosci 10(5):512–526. https://doi.org/10.1080/17470919.2015.1087426
Kiyokawa Y, Takeuchi Y (2017) Social buffering ameliorates conditioned fear responses in the presence of an auditory conditioned stimulus. Physiol Behav 168:34–40. https://doi.org/10.1016/j.physbeh.2016.10.020
Borges JV, de Freitas BS, Antoniazzi V, dos Santos CdS, Vedovelli K, Pires VN, Paludo L, Martins de Lima MN et al (2019) Social isolation and social support at adulthood affect epigenetic mechanisms, brain-derived neurotrophic factor levels and behavior of chronically stressed rats. Behav Brain Res 366:36–44. https://doi.org/10.1016/j.bbr.2019.03.025
Ma DY, Chang WH, Chi MH, Tsai HC, Yang YK, Chen PS (2016) The correlation between perceived social support, cortisol and brain derived neurotrophic factor levels in healthy women. Psychiatry Res 239:149–153. https://doi.org/10.1016/j.psychres.2016.03.019
Furtbauer I, Heistermann M (2016) Cortisol coregulation in fish Sci Rep 6:30334. https://doi.org/10.1038/srep30334
Liu H, Yuan TF (2016) Physical interaction is required in social buffering induced by a familiar conspecific. Sci Rep 6:39788. https://doi.org/10.1038/srep39788
Li LF, Yuan W, He ZX, Wang LM, Jing XY, Zhang J, Yang Y, Guo QQ et al (2019) Involvement of oxytocin and GABA in consolation behavior elicited by socially defeated individuals in mandarin voles. Psychoneuroendocrinology 103:14–24. https://doi.org/10.1016/j.psyneuen.2018.12.238
Mirrione MM, Schulz D, Lapidus KA, Zhang S, Goodman W, Henn FA (2014) Increased metabolic activity in the septum and habenula during stress is linked to subsequent expression of learned helplessness behavior. Front Hum Neurosci 8:29. https://doi.org/10.3389/fnhum.2014.00029
Chou D, Peng HY, Lin TB, Lai CY, Hsieh MC, Wen YC, Lee AS, Wang HH et al (2018) (2R,6R)-hydroxynorketamine rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology 139:1–12. https://doi.org/10.1016/j.neuropharm.2018.06.033
Strong PV, Christianson JP, Loughridge AB, Amat J, Maier SF, Fleshner M, Greenwood BN (2011) 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience 197:132–144. https://doi.org/10.1016/j.neuroscience.2011.09.041
Diao Z, Yao L, Cheng Q, Wu M, Di Y, Qian Z, Wei C, Liu Y et al (2021) Involvement of midbrain dopamine neuron activity in negative reinforcement learning in mice. Mol Neurobiol. https://doi.org/10.1007/s12035-021-02515-6
Okon-Singer H, Hendler T, Pessoa L, Shackman AJ (2015) The neurobiology of emotion-cognition interactions: fundamental questions and strategies for future research. Front Hum Neurosci 9:58. https://doi.org/10.3389/fnhum.2015.00058
Smith ML, Asada N, Malenka RC (2021) Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science 371(6525):153–159. https://doi.org/10.1126/science.abe3040
Peen NF, Duque-Wilckens N, Trainor BC (2021) Convergent neuroendocrine mechanisms of social buffering and stress contagion. Horm Behav 129:104933–104933. https://doi.org/10.1016/j.yhbeh.2021.104933
Abraham E, Raz G, Zagoory-Sharon O, Feldman R (2018) Empathy networks in the parental brain and their long-term effects on children’s stress reactivity and behavior adaptation. Neuropsychologia 116(Pt A):75–85. https://doi.org/10.1016/j.neuropsychologia.2017.04.015
Lee IC, Yu TH, Liu WH, Hsu KS (2020) Social transmission and buffering of hippocampal metaplasticity after stress in mice. J Neurosci. https://doi.org/10.1523/JNEUROSCI.1751-20.2020
Acknowledgements
We appreciated Qian Zhang and Feng He for their technical assistant.
Funding
This work was supported financially by grants from the National Natural Science Foundation of China (No. 31571044, 31871073 to B.T., and No. 31600821 to P.Z.), Program for New Century Excellent Talents in University (No. NCET- 10–0415 to B.T.), and the Fundamental Research Funds for the Central Universities (HUST: 2019kfyXJJS081 to P.Z.).
Author information
Authors and Affiliations
Contributions
This study was designed by Bo Tian, Jie Ming, Pei Zhang, and Hongwei Cai. The experiments were complemented by Hongwei Cai, Guangjian Qi, Lijun Zhang, Ming Li, Xinyuan Lv, and Jie Lei. Statistical analysis was performed by Hongwei Cai and Tongxia Li. Hongwei Cai and Pei Zhang finished manuscript writing. Bo Tian, Jie Ming, and Pei Zhang provide the supervision and final check.
Corresponding authors
Ethics declarations
Ethics Approval
All animal studies were performed in accordance with ethical guidelines and all procedures were approved by the Animal Care and Use Committee (Laboratory animal center of Huazhong University of Science and Technology, Wuhan, China).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cai, H., Zhang, P., Qi, G. et al. Systematic Input–Output Mapping Reveals Structural Plasticity of VTA Dopamine Neurons-Zona Incerta Loop Underlying the Social Buffering Effects in Learned Helplessness. Mol Neurobiol 59, 856–871 (2022). https://doi.org/10.1007/s12035-021-02614-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02614-4