Abstract
Electroacupuncture (EA) is considered to have potential antidiabetic effects; however, the role of the pancreatic intrinsic nervous system (PINS) in EA-induced amelioration of type 2 diabetes (T2DM) remains unclear. Therefore, we investigated whether EA at ST25 exerts any beneficial effects on insulin resistance (IR), inflammation severity, and pancreatic β cell function via the PINS in a rat model of a high-fat diet-streptozotocin (HFD/STZ)-induced diabetes. To this end, Sprague Dawley rats were fed with HFD to induce IR, followed by STZ (35 mg/kg, i.p.) injection to establish the T2DM model. After hyperglycemia was confirmed as fasting glucose level > 16.7 mmol/L, the rats were treated with EA (2 mA, 2/15 Hz) for the next 28 days. Model rats showed increased serum glucose, insulin, IR, and TNF-α levels with a concomitant decrease in β cell function. Microscopy examination of the pancreas revealed pathological changes in islets, which reverted to near-normal levels after EA at ST25. EA improved islet cell morphology by increasing islet area and reducing vacuolation. EA at ST25 decreased transient receptor potential vanilloid 1 (TRPV1) and increased substance P (SP) and calcitonin gene-related peptide (CGRP) expression. Subsequently, insulin secretion decreased and impaired pancreatic endocrine function was restored through the TRPV1 channel (SP/CGRP)-insulin circuit. EA increased choline acetyltransferase and neuropeptide Y expression and controlled inflammation. It also enhanced the cocaine and amphetamine-regulated transcript prepropeptide expression and promoted glucagon-like peptide-1 secretion. Additionally, the electrophysiological activity of PINS during acupuncture (2.71 ± 1.72 Hz) was significantly increased compared to the pre-acupuncture frequency (0.32 ± 0.37 Hz, P < 0.05). Thus, our study demonstrated the beneficial effect of EA on β cell dysfunction via the PINS in rat models of HFD-STZ-induced T2DM.
Similar content being viewed by others
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) has been rapidly increasing worldwide. According to the latest reports, the total number of patients with diabetes in mainland China is estimated to be 129.8 million [1]. T2DM is strongly associated with obesity [2] and characterized by progressive pancreatic β cell dysfunction [3], accompanied by insulin resistance (IR) [4]. IR contributes to impaired glucose homeostasis and type 2 diabetes [5, 6]. Pancreatic β cell dysfunction is central to the pathogenesis of type 2 diabetes [7]. Preserving β cell function during the development of obesity and IR would limit the development of type 2 diabetes [8]. Meanwhile, being overweight increases the risk of metabolic disease; more than a third of the Chinese population has prediabetes [9]. Recent studies have demonstrated that acupuncture could regulate lipid metabolism disorder [10] and improve glucose tolerance (IGT) [11], which is beneficial to the prevention and treatment of T2DM. Acupuncture is effective in the management of various metabolic disorders such as hyperglycemia and overweight by alteration of the sympathetic nervous system and insulin signal defects [12]. Acupuncture has been practiced in East Asian countries to relieve a variety of illnesses and is now widely accepted worldwide [13]. It can facilitate weight control by regulating the nervous, endocrine, and digestive systems [14]. It might also be useful in reducing blood glucose levels in patients with T2DM [15] and improving insulin sensitivity [16]. When combined with metformin, the treatment, including electroacupuncture (EA) at ST25, can be used as an insulin sensitizer to effectively manage the risk of T2DM and obesity [17]. While recent studies confirmed that EA alone can also attenuate blood glucose. EA could effectively ameliorate adipose accumulation of obese men [18]. Stimulating bilateral ST25 could effectively regulate fasting blood glucose, insulin, and lipid metabolism [19]. EA might be an alternative for managing islet function and treating T2DM. However, the mechanisms by which acupuncture regulates islet functions remain to be elucidated.
A potential mechanism for the hypoglycemic effect of acupuncture is a neuroendocrine pathway involving crosstalk among the endocrine, nervous, and immune systems. EA modulates distinct sympathetic pathways. Acupuncture at body surface points can mediate the activities of a variety of somatosensory, autonomic, and target organ reflex pathways. Therefore, EA may influence changes in systemic metabolism. We have previously confirmed that EA at ST25 can regulate the activity of glucose-inhibited neurons and improve lipid metabolism disorders [20]. However, the local related neurological changes of the pancreas and the onset mechanism of EA in T2DM are still unclear. Recent work suggests that the pancreatic intrinsic nervous system (PINS) is involved in glucose homeostasis, insulin sensitivity, and pancreatic β cell function, and thus the pathogenesis of diabetes [21]. It suggested that the intrinsic nervous system is one of the neural mechanisms of acupuncture regulating blood glucose.
Intrapancreatic ganglia constitute a complex information-processing center that contains various neurotransmitters and forms an endogenous neural network, which has a major influence on pancreatic endocrine function; those neurotransmitters including choline acetyltransferase (ChAT) and neuropeptide Y (NPY) control inflammatory status, cocaine, and amphetamine-regulated transcript (CART-PT) to promote the secretion of glucagon-like peptide-1 (GLP-1) and a promising axis in TRPV1 channel (SP/CGRP)-insulin circuit [22].
EA can control the inflammatory status and have a positive significance for the management of T2DM. EA at ST25 reduced patients’ inflammation levels, thereby improving insulin sensitivity [23]. While the spinal sympathetic axis evoked by 3 mA EA at ST25 can suppress splenic inflammation [24]. The vagus nerve splits in the celiac ganglion, giving rise to the postganglionic splenic nerve that terminates in the spleen [25]. The spatial resolution of the sensations that can be elicited from the viscera is relatively vague and can be fully explained by the segmental width of the afferent inflow from each viscus. Similar neuromeric segments are also present in the pancreas [26, 27]. ST25 is innervated by T10 [28], whose ganglion segment partially overlaps with the pancreas (innervated by T5-T11 [29, 30]). EA at ST25 can reduce pancreatic inflammation via restraint inflammatory factor and NK-κB [31]. The balance of pro- and anti-inflammatory factors in pancreas can be ameliorated by EA at ST25 [32]. Consequently, we hypothesized that EA at ST25 will lead to a hypoglycemic effect through neural regulation of pancreatic endocrine secretion. We tested this hypothesis in the study presented here.
Materials and Methods
Establishment of the Experimental Animal Model
The use of a combination of a high-fat diet (HFD) and a low dose of streptozotocin (STZ) has been shown to effectively establish a rat model of diabetes that mimics the metabolic characteristics of common T2DM in humans [33]. Seven-week-old Sprague Dawley (SD) rats were supplied by the model animal research center of the Nanjing University of Chinese Medicine (No. 1100112011052760, under grant SCXK(JING)2016-0006). The experimental rats were maintained in a controlled environment (conditions: 12-/12-h ± 1-h light/dark cycle; temperature, 22 ± 2 °C; relative humidity 60% ± 5%). The animals were raised in individual cages with ad libitum access to food and water and randomly numbered. They were divided into three groups: the model, EA, and control groups, with six animals each. T2DM was induced by administering a high-fat diet and low-dose STZ (HFD-STZ). The model and EA groups were fasted for 16 h and intraperitoneally (i.p.) injected with STZ (Sigma-Aldrich, St. Louis, MO, USA) dissolved in freshly prepared citrate buffer (0.1 mol/L, pH 4.2) at a dose of 35 mg/kg. Random blood glucose levels were measured 48 h after STZ injection. Rats with a random blood glucose level of >16.7 mmol/L and kept for 2 weeks were considered rats with T2DM. Weight, food intake, and random blood glucose levels were recorded weekly. Random blood glucose levels were monitored weekly by collecting blood from the tail vein and analyzing it with a glucometer (Roche Diagnostics, Mannheim, Germany). To induce IR, the model and EA groups were placed on an HFD [34] consisting of 58% fat, 25% protein, and 17% carbohydrate, as a percentage of total kcal upon arrival, which was maintained for the duration of the study. The control group was fed a standard normal chow diet. The remaining non-T2DM rats in the model and EA groups were killed by cervical dislocation. The schedule of the experimental procedures is shown in Fig. 1. All the experiments were performed per the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals published by the National Science Council, China (under grant 202006A016).
Blood and Tissue Sample Collection
At the end of the experiment (week 12), the rats fasted for 12 h and anesthetized with isoflurane. Blood samples were drawn from the orbital sinus and centrifuged at 3000 rpm for 15 min at 4 °C. The separated serum was stored at −80 °C for further procedures. The pancreas and duodenum were quickly removed, rinsed, and stored at −80 °C or fixed in 10% paraformaldehyde solution. The metabolic metrics including fasting serum insulin, hemoglobin A1c, leptin, and GLP-1 levels and those of the pro-inflammatory cytokines including tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and IL-1β, and anti-inflammatory cytokines such as interleukin 10 (IL-10) in the serum were determined using rat ELISA kits (Nanjing Jiancheng Bioengineering Institute Co., Ltd.) according to the manufacturer’s instructions.
Antibodies
Membranes were blocked and probed with primary and secondary antibodies according to the manufacturers’ suggested concentrations. The primary antibodies used are listed in Table 1. The secondary antibodies used were anti-rabbit IgG, HRP-linked antibody (1:2000, Cell Signaling Technology), and anti-mouse IgG, HRP-linked antibody (1:2000, Cell Signaling Technology).
Measurement of Random Blood Glucose Levels
Random blood glucose levels were recorded weekly using an Accu-Chek glucometer (Roche Diagnostics, Mannheim, Germany). Animals were free to eat and drink. Homoeostasis model assessment (HOMA) was used to estimate basal β cell function (HOMA-β) and insulin resistance (HOMA-IR). HOMA-β was calculated as follows: 20 × fasting insulin (FINS)/fasting plasma glucose (FPG)—3.5. HOMA-IR was calculated as FPG × FINS/22.5 [35].
Intravenous Glucose Tolerance Test
Acute insulin secretory response was measured by performing the intravenous glucose tolerance test (IVGTT). Overnight, fasted rats from all the groups were subjected to an oral glucose tolerance test in the last week of the experimental period. The blood glucose levels were monitored at 0, 30, 60, 90, and 120 min by using an Accu-Chek glucometer (Roche Diagnostics, Mannheim, Germany) after intraperitoneal administration of 2 g/kg b.w./rat glucose as an aqueous solution [36].
Hematoxylin and Eosin Staining
Hematoxylin and eosin (HE) staining was performed according to standard histological protocols. After being carefully isolated, the pancreas was fixed in 4% paraformaldehyde and embedded in paraffin wax. Then, 8-μm-thick sections were obtained with a rotary slicer (Leica, Germany) and mounted on slides. HE staining was performed under a light microscope (Olympus, Japan) to visualize pathological changes.
Western Blot Analysis
Pancreas tissue (200 mg in weight) was obtained from the animals under anesthesia (0.8 g/kg urethane, i.p.) after 12 h of fasting. The tissue (100 mg) was placed in 1 mL of lysis buffer consisting of protease inhibitor and RIPA buffer (Thermo Fisher Scientific), homogenized, and centrifuged at 14,000 rpm for 30 min. After that, protein concentrations were measured with the BCA Protein Assay Kit (Thermo Fisher Scientific). Then, 20 μg of protein from each sample was resolved electrophoretically on sodium dodecyl sulfate-polyacrylamide gel (12% of separation gel and 5% of concentration gel). Electrophoresis was performed at 70 V for 0.5 h and 110 V for 1 h. The protein bands were transferred to polyvinylidene difluoride membranes using Trans-Blot Turbo Transfer System (Bio-Rad). Bovine serum albumin (5%) was added for 2 h to block the membranes. Thereafter, each membrane was further blocked using 5% bovine serum albumin for 1 h and then washed with Tris-buffered saline containing Tween (TBST). The primary antibodies were diluted as listed in Table 1. The tissues were incubated with each antibody at 4 °C for 16 h overnight. Then, the membranes were rewashed with TBST and incubated with the secondary antibodies. After incubating with the corresponding secondary antibodies at 28 °C for 1 h, the membranes were analyzed by enhanced chemiluminescence detection. The gray values of the immunoreactive protein bands were quantified using Image J (NIH, Bethesda, MD, USA).
Immunofluorescence Staining
Frozen sections were used for immunofluorescence (IF) staining. Pancreas tissue was fixed in 4% paraformaldehyde overnight and dehydrated in 30% sucrose in 0.1 M PBS (Biosharp Life Sciences, China) at 4 °C. After embedding in optimal cutting temperature compound, the pancreas tissue was sliced into 10-μm-thick sections and mounted on slides. The sections were then blocked in 0.2% Triton X-100 (Sigma-Aldrich (Shanghai) Trading Co., Ltd.) for 10 min and permeabilized in Sea BLOCK Blocking Buffer (Thermo Fisher Scientific, USA) for 1 h. They were then incubated with primary antibodies against insulin (1:100, SAB) overnight at 4 °C, followed by incubation with secondary antibodies Alexa Fluor 488 (goat anti-rabbit, 1:500, Abcam, Cambridge, UK), Alexa Fluor 594 (goat anti-mouse, 1:500, Abcam, Cambridge, UK), or Alexa Fluor 405 (Goat anti-rabbit, 1:500, Abcam, Cambridge, UK) for 1 h at 37 °C. Different combinations of secondary antibodies were used to obtain optimal images. Finally, the tissue sections were covered by coverslips after washing them with 0.1 M PBS. Images were obtained by a fluorescence microscope (Olympus BX60 Darkfield DIC Metallurgical Microscope, Japan).
Recording of Electrophysiological Activity in PINS
In order to obtain the discharge of the pancreatic intrinsic nervous system, the rats were anesthetized with isoflurane inhalation (2–5%) via a precision vaporizer (RWD Life Science Co., Ltd., Shenzhen, China). The depth of anesthesia was assessed by the absence of corneal and hind paw withdrawal reflexes. A laparotomy incision of approximately 3 cm was made in the skin just to the right of the abdomen midline. Dissection of the longitudinal muscle with adherent myenteric plexus has been dissected from the duodenum, leaving intact the connective tissue between the bowel and pancreas [37]. The pancreatic nerves run along the splenic artery and superior and inferior pancreatic arteries [38, 39]. The branch of the pancreatic intrinsic nerve was separated and connected to the positive electrode (PFA-Coated Platinum, A-M Systems, USA, 772000). The reference electrode was attached to the surrounding tissue. The experimental rats are placed in Faraday cages to shield them from electromagnetic interference signals. To avoid signal interference, only the electrodes that touch the nerves were exposed [40]. Spikes were recorded using a preamplifier (A-M Systems, Carlsborg, WA, USA; band-passing: 10–1000 Hz, sampling frequency: 20,000 Hz, and amplification: 1000-fold) and connected to a biosignal acquisition and analysis system (Microl 1401-3, CED, UK). The data were analyzed with Spike2 software.
Acupuncture Intervention
EA refers to the application of a pulsating electrical current to acupuncture needles for acupoint stimulation [41]. The rats in the EA group received EA treatment on bilateral ST25 (Tianshu, located 5 mm lateral to the intersection between the upper 2/3rd and the lower 1/3rd in the line joining the xiphoid process and the upper border of the pubic symphysis) after gas anesthesia with isoflurane (2–5%). Meanwhile, the same anesthesia was administered to rats in the model group but without performing EA. For the EA group, two stainless steel acupuncture needles (Hwato, 20162270970, Suzhou, China) of 0.2 mm in diameter were inserted at a depth of 5 mm into the ST25 acupoint. EA at ST25 was conducted with the HANS-100A (Han Acuten, WQ1002F, Beijing, China) apparatus set to a current of 2 mA and a frequency of 2/15 Hz. All acupuncture procedures were performed by an experienced and licensed acupuncturist: 30 min a day for 6 days a week, 1 week a course, over four continuous courses of treatment.
Data Analysis
Data from all the experiments are expressed as mean ± standard error values. Paired t-test was used for comparison before and after EA intervention, and an independent t-test was used for comparison between the two different groups. Multiple group comparisons were conducted using one-way ANOVA. All data analyses were performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA), and GraphPad Prism 8.0 (GraphPad Inc., La Holla, CA, USA) was used for data analysis. p < 0.05 was considered to indicate statistical significance.
Results
EA at ST25 Can Decrease Blood Glucose Level and Weight and Improve IVGTT
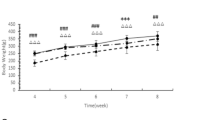
The model group’s blood glucose level was higher than that of the control group (P < 0.05) from the second week and continued to increase, as shown in Fig. 2a. The model group’s weight was lower than the control group from the fifth week (P < 0.05), as shown in Fig. 2b. The hyperglycemia and the high body weight of the model group were maintained during the treatment, which lasted for 4 weeks. The blood glucose level and weight gain in the EA group decreased from the second week of treatment. These changes were statistically significant in comparison with those in the model group (P < 0.05). Moreover, these effects persisted for 3 weeks until the end of the experiment.
a Random blood glucose levels of rats during the experiment. b Weight of rats during the experiment. c Area under the curve (AUC) for the IVGTT test. d IVGTT test results after treatment. Results were expressed as mean ± SEM (n = 6). Data were analyzed by one-way ANOVA; paired t-test was used for comparison before and after EA intervention; *p < 0.05 vs. control, #p < 0.05 vs. model
The glucose tolerance test was more sensitive than FPG alone in diagnosing T2DM. FPG or random blood glucose levels could not be used as a comprehensive measure of glycemic control. Intravenous glucose tolerance test (IVGTT) was performed at the end of week 8 (the last week before EA treatment) as well as week 12 (the last week of the experiment) to show the curative effect of EA. Additionally, the area under the curve (AUC) for the IVGTT test was computed as a measure of total glucose exposure. Figure 2c reveals that 4 weeks of EA improved glucose tolerance in rats. The AUC of the EA group was significantly lower than that of the model group (P < 0.05). For the EA group, the AUC after treatment was smaller than that before EA (P < 0.05, not labeled). Moreover, a small rise in the AUC in the model group was also observed, which suggests that the condition of the untreated rats continued to deteriorate. Furthermore, Fig. 2d shows the results of the IVGTT after treatment. The blood glucose level in the EA group decreased from 60 to 120 min and was lower than that in the model group (P < 0.05).
EA at ST25 Improved Insulin Sensitivity and Controlled Inflammatory Status
The maintenance of normal blood glucose levels is critical for the body to function properly. Markers of carbohydrate metabolism, such as glucose and insulin, are strongly associated with health problems in HFD-STZ rats. Data showing a comparison of β cell function and IR among groups can be found in Fig. 3. Insulin plays a key role in controlling blood glucose levels. The FPG and insulin of the model rats were significantly higher than that in the control rats (P < 0.01, Fig. 3a and b). EA at ST25 resulted in a decrease in FPG and insulin in comparison with the values in model rats (P < 0.01). Accordingly, HOMA-IR of the model rats was significantly higher than that in the control rats (P < 0.01, Fig. 3c), while EA at ST 25 led to a decrease in HOMA-IR when compared to model rats (P < 0.01). Remarkably, the HOMA-β of the model rats was much lower than in the control rats (P < 0.01, Fig. 3d). However, the EA group showed a higher HOMA-β than the model group (P < 0.01). Thus, the glucose metabolism of rats was normalized to a degree after EA but did not completely recover.
Comparison of β cell function and insulin resistance among groups. Fasting plasma glucose, FPG (a), fasting serum insulin, INS (b), HOMA-IR (c), HOMA-β (d), hemoglobin A1c, HbA1c (e), leptin, LEP (f), glucagon-like peptide-1, GLP-1 (g), tumor necrosis factor-α, TNF-α (h), IL-1β (i), and IL-10 (j). Results were expressed as mean ± SEM (n = 6). Data were analyzed by one-way ANOVA. *p < 0.05 vs. control, #p < 0.05 vs. T2DM
For decades, hemoglobin A1c (HbA1c) has remained the standard biomarker for glycemic control [42]. The HbA1c level of the model rats was higher than that in the control rats (P < 0.01, Fig. 3e). EA at ST25 decreased the HbA1c level in comparison with that in model rats (P = 0.114). Leptin, a hormone secreted from adipose tissue, plays a key role in energy balance and feeding behavior through neuronal regulation. Both leptin deficiency and leptin resistance are associated with the development of obesity [43]. The leptin level in the model group was higher than that in the control group (P < 0.01, Fig. 3f), while the leptin level in the treatment group was lower than that in the model group (P < 0.01). GLP-1 is a peptide hormone secreted from enteroendocrine L-cells into the hepatic portal circulation in response to ingestion of nutrients [44]. The GLP-1 level in the model group was lower than that in the control group (P < 0.01, Fig. 3g), while the GLP-1 level in the treatment group was higher than that in the model group (P < 0.01). T2DM is a polygenic disease with a low-grade inflammatory component. TNF-α is an inflammatory cytokine produced by various cells, including immune cells and epithelial cells [45]. Interleukin-1β (IL-1β) has been reported to contribute to β cell failure, and therapies targeting IL-1β have shown encouraging progress, albeit with diverse results in different clinical trials [46]. TNF-α and IL-1β of the model group were significantly higher than that of the control group (P < 0.05, Fig. 3h and i). EA at ST25 resulted in a decrease in TNF-α and IL-1β compared to model rats (P < 0.05). IL-10 is an anti-inflammatory cytokine that is known to suppress effector T cell responses and limit inflammation [47]. IL-10 level in the model group was lower than that in the control group (P < 0.05, Fig. 3j). The IL-10 level in the treatment group was higher than that in the model group (P < 0.05).
Restoration of Islet Morphology Through Pancreatic Intrinsic Nervous System by EA
Histological analysis was performed to observe islet morphology (Fig. 4). The pancreatic islet area of normal control rats revealed a normal architecture without any β cell damage. The morphology of islets showed a preserved round shape in the control group (Fig. 4a and d). In contrast, HFD-STZ-induced diabetic rats showed moderate damage and swelling of pancreatic β cells (Fig. 4b and e). Hyperglycemia led to marked changes in islet morphology [48]. The edges were irregular, the islet area was decreased, and the cells contained significantly more vacuoles. The islet shape was regular in the EA group (Fig. 4c and f). The morphology of islets in the ST25 group closely resembled that of the intact islets. EA improved the islet cell morphology by increasing the islet area, reducing vacuolation compared to those of the model group.
Representative HE and IF images of the pancreas. Green arrowheads show islets. Red arrowheads represent vacuoles. DAPI stained the nuclei (blue), and the green immunofluorescence represents the insulin. Islets were observed under a microscope (×400 magnification). Scale bar = 50 μm. The three groups share scale bars
The pancreatic intrinsic nervous system was damaged during T2DM (Fig. 5h and k). The pan-neuronal marker protein gene product 9.5 (PGP9.5) was first examined by WB to explore the hypoglycemic effect of electroacupuncture at ST25 through neural regulation of pancreatic endocrine secretion. The expression of PGP9.5 in the pancreatic tissue of the model rats was significantly lower than that in the control rats (P < 0.05, quantification of PGP9.5 was checked by WB and is shown in Fig. 6). EA at ST25 resulted in an increase in PGP9.5 expression in comparison with model rats (P < 0.05). These findings were validated with the results of another experiment as described below (Fig. 6).
Representative IF images of the pancreas. DAPI stained the nuclei (blue), while the red immunofluorescence represents the insulin, and the green immunofluorescence represents the PGP9.5. The yellow color in the merged pictures indicates co-expression. Islets were observed under a microscope (×400 magnification). Scale bar = 50 μm. The three groups share scale bars
More research has been performed to identify specific neurotransmitters that can be modulated through EA at ST25. Additionally, the levels of ChAT, CGRP substance P (SP), NPY, and CART-PT expression decreased in the model group (P < 0.05), indicating PINS remodeling. The expression of these neurotransmitters improved after EA at ST25, as shown in Fig. 6.
Pancreatic Endocrine Function Was Restored Through the TRPV1-(SP/CGRP)-β Cell Circuit
For further investigations, we also observed the expression of neural markers of sensory neurons has been observed, too. We focused on the expression of CGRP and SP since they can be regulated through transient receptor potential vanilloid 1(TRPV1) [49]. On the basis of the findings obtained with the observation of insulin in rat pancreas, we further evaluated the results of immunofluorescence staining and identified the expression of TRPV1 and insulin in rat pancreas to explore the neural regulation of pancreatic endocrine secretion through EA (Fig. 7a). In comparison with the model control group, the model group showed an increased fluorescently stained area of TRPV1 and a reduced area in the EA group. Quantification of TRPV1, CGRP, and SP in rat pancreas was performed by WB and shown in Fig. 7b.
a Immunofluorescence staining and identification of TRPV1 (green) and insulin (blue) expression in the rat pancreas. Islets were observed under a microscope (×400 magnification); b The effect of EA on the expression of vanilloid 1 (TRPV1), CGRP, and SP. Vinculin was used as an internal reference protein. *p < 0.05 vs. control, #p < 0.05 vs. model
Electrophysiological Activity of PINS Before/After ST25 Acupuncture
In order to clarify the connections between ST25 and PINS, we then examined discharges of the PINS in normal rats. The activity of PINS during MA (2.71 ± 1.72 Hz) was significantly increased compared to the pre-MA frequency (0.32 ± 0.37 Hz, P < 0.05, Fig. 8a). The anatomical location of PINS is shown in Fig. 8b.
The effect of manual acupuncture at Tianshu (ST25) acupoint on the activity of PINS. a Waveform of PINS discharge by MA at ST25. b The anatomical location of PINS. The blue line represents PINS, which travels from the duodenum to the pancreas. The red lines show the blood vessels between the pancreas and duodenum (modified from Zoucas E 1996). MA, manual acupuncture; PINS, pancreatic intrinsic nervous system
Discussion
T2DM is characterized by IR combined with progressive pancreatic β cell failure [50], which can be moderated by acupuncture. The abnormal glycolysis process can affect lipid metabolism disorder, forming a vicious cycle [51], causing and aggravating oxidative stress, nerve damage, and other pathological changes [52]. In patients with diet-induced obesity, excess metabolites and lipid consumed in the diet result in high levels of circulating leptin, and the dysregulated leptin signaling maintains adipocyte hypertrophy and obesity [53]. Exogenous GLP-1 infusion increased plasma GLP-1 concentrations and caused a transient, but non-sustained, suppression of glucagon [54]. EA at ST25 can reduce HOMA-IR and increase HOMA-β. Specifically, after EA at ST25, the levels of fasting blood glucose, insulin, and glycated hemoglobin were all lower than those in the model group, while the level of GLP-1 increased. CART stimulates intestinal glucagon in a pathway independent of the known GLU Tag and STC-1 pathways [55] and increases Ca2+ signal transduction in the islet [56] in addition to altering islet β cells morphology [57]. Thus, the CART system may be an emerging therapeutic target for T2DM. In conclusion, as a GLP-1 regulator, CART can indirectly participate in the glucose metabolism of the pancreas. Additionally, EA at ST25 elevated the expression of leptin, as shown in Fig. 3, and leptin can also independently lower blood glucose levels [58].
EA had differential effects on metabolic markers in the HFD-STZ-induced rat model of T2DM. These effects may be explained neuroanatomically by variations in the segmental innervation of tissues at these locations [59, 60]. The acupuncture treatment reduced the HbA1c level significantly in comparison with that in the sham-acupuncture group [61]. The effectiveness of acupuncture in treating diseases related to IR had been reported, and acupuncture had an advantage over control groups in decreasing fasting blood glucose (FBG) and fasting insulin levels [62, 63]. Preventive acupuncture is beneficial for the control of STZ-induced hyperglycemia in rats [64]. EA intervention can significantly protect islet function and improve the FBG level in T2DM via regulation of thyroid hormone and phosphatidylinositol signaling [65].
As mentioned before, PINS constitute a complex information-processing center that includes various neurotransmitters and forms an endogenous neural network, which has an important influence on pancreatic endocrine function. PGP and multiple neurotransmitters have been shown to be upregulated by EA at ST25, suggesting adaptive changes in pancreatic-related nerves and transmitters. Those phenomena provided a neurophysiological basis for the onset of EA.
Chronic inflammation is closely related to pancreatic β cell damage [66]. EA at ST25 ameliorated the inflammatory state, contributing to the restoration of pancreatic β cell morphology (Fig. 4). Consistent with Ma’s research [16], the expression of NPY can explain the related experimental effects, including the anti-inflammatory effects. In addition, the changes in NPY expression over time showed the anti-inflammatory effect of EA at ST25, which can be observed in Fig. 3. In terms of inflammatory markers, the expression of TNF-α and IL-1β was decreased, while the expression of anti-inflammatory IL-10 was increased. The accumulation of pro-inflammatory factors can disturb the balance of apoptosis [67] and inhibit cell growth [68]. The anti-inflammatory effect of EA also indirectly protected β cells from apoptosis. Additionally, low-frequency electrical stimulation of the efferent vagal nerve fibers is thought to possess anti-inflammatory properties and can activate the “cholinergic anti-inflammatory reflex” [69]. ChAT is the most suitable factor for monitoring cholinergic neurons [70]. The increased expression of these neurotransmitters was consistent with the inflammatory activity, which verified the anti-inflammatory effect of EA at ST25.
Further explanation of our study focuses on the restoration of pancreatic endocrine function by EA at ST25 through the TRPV1 channel (SP/CGRP)-β cell circuit. The TRPV1 channel is highly expressed on sensory nerve fibers innervating the pancreas [71]. TRPV1 can be upregulated by high glucose levels [72], as observed in our study (Fig. 7b). It confirmed that TRPV1 was involved in the regulation of glucose. The pancreas receives sensory innervation, and its axon endings are sensitive to capsaicin and can release CGRP locally, which can induce diabetes [73].
In rodent models of T2DM, TRPV1 signal transduction is activated, and SP and CGRP release is increased chiefly. SP can inhibit glucose-induced insulin release and reduce glucose uptake, and thus improve insulin resistance [74]. However, we found that the expression of SP decreased in HFD-STZ-induced T2DM rats as the islets showed ill-conditioned patterns. This conclusion is consistent with Razavi’s assertion [75]. SP is one of the vital sources of pancreatic duct proliferation [76]. Pancreatic duct cells show the physiological characteristics of stem cells, which are differentiated into islet β cells [77]. Low levels of SP will lead to the proliferation of β cells and change glucose homeostasis [78]. Additionally, SP inhibits insulin secretion at low levels and promotes it at high levels [79]. In addition to TRPV1-expressing neurons, SP/CGRP also exists in islet cells and inhibits the release of insulin levels by β cells [80, 81]. Collectively, β cell activity was out of control as a result of deficient SP and CGRP.
Under physiological conditions, the functioning of TRPV1 will be affected by β cells. Chemical ablation of TRPV1 neurons can affect the function and quantity of islet β cells and improve glucose metabolism, indicating that β cells are vital targets of TRPV1 neurons [82]. The decrease in SP and CGRP can further activate the TRPV1 receptor through a positive feedback loop. However, there is a physiological limit to the expression level of TRPV1 receptors [32]. TRPV1 receptors degenerate after overstimulation, and although they remain at a high level, their sensitivity to stimulation decreases. SP/CGRP lacks the activation of the corresponding receptors, so it presents a low-level pathological state. Eventually, insulin will accumulate excessively, leading to the development of IR. This kind of TRPV1 channel (SP/CGRP)-β cell circuit balance disorder can lead to hyperinsulinemia, induce systemic IR, and disrupt glucose homeostasis.
In this study, we found that EA at ST25 could remarkably reduce IR and partially restore β cell function in T2DM rats. Further analysis showed that ST25 stimulation restores vital pancreatic functions regulated by PINS, regardless of pancreatic health, with transmitters such as NPY playing a critical role in this effect. Therefore, the significantly decreased HOMA-IR caused by stimulation at ST25 may be mediated via nervous innervation of the acupoint areas and imply the role of the PINS. WB and IF analyses showed that protein expression of PGP9.5 returned to nearly control levels after EA stimulation at ST25.
However, the islets of Langerhans make up only about 2% of the mass of the pancreas, so extrapolating the results from the whole pancreas to the endocrine pancreas is difficult. Although TRPV1 and CGRP/SP are of substantial significance in the treatment of T2DM [59], the neuroendocrine communication mechanism formed by TRPV1 and CGRP/SP is still unclear and needs further study.
Conclusions
Current therapeutic strategies to manage hyperglycemia do not halt (or reverse) disease progression and may even cause undesirable adverse effects and comorbidities on their own [83], while treatment with insulin, sulfonylureas, and glinides may lead to weight gain [84]. EA at ST25 can reduce the body weight of T2DM rats and improve glucose metabolism. Using a high-fat-fed, STZ rat model that shows the metabolic characteristics of human T2DM, our study tested the hypothesis that EA at ST25 would repair the pancreas after STZ injury through neural regulation of the pancreatic intrinsic nervous system.
References
Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H et al (2020) Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ 369:m997
Cheon J, Kim D, Kim K-S (2015) Insulin sensitivity improvement of fermented Korean Red Ginseng ,Panax ginseng,mediated by insulin resistance hallmarks in old-aged ob/ob mice. J Ginseng Res 39:331–337
Campbell RK (2009) Fate of the beta-cell in the pathophysiology of type 2 diabetes. J Am Pharm Assoc 49:S10–S15
Freeman Jeffrey S (2010) A physiologic and pharmacological basis for implementation of incretin hormones in the treatment of type 2 diabetes mellitus. Mayo Clin Proc 85:S5–S14
Samuel Varman T, Shulman Gerald I (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148:852–871
Biddinger SB, Kahn CR (2006) From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol 68:123–158
Prentki M, Nolan CJ (2006) Islet beta cell failure in type 2 diabetes. J Clin Invest 116:1802–1812
Marmugi A, Parnis J, Chen X, Carmichael L, Hardy J, Mannan N et al (2016) Sorcin links pancreatic β-cell lipotoxicity to ER Ca2+ stores. Diabetes 65:1009–1021
Pan XF, Wang L, Pan A (2021) Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol 9:373–392
Lu M, He Y, Gong M, Li Q, Tang Q, Wang X et al (2020) Role of neuro-immune cross-talk in the anti-obesity effect of electro-acupuncture. Front Neurosci 14:151
Li X, Liu H, Feng H, Xian Z, Chen Y, Chen J et al (2017) Acupuncture paired with herbal medicine for prediabetes: study protocol for a randomized controlled trial. Trials 18:297
Liang F, Koya D (2010) Acupuncture: is it effective for treatment of insulin resistance? Diabetes Obes Metab 12:555–569
Kim DH, Ryu Y, Hahm DH, Sohn BY, Shim I, Kwon OS et al (2017) Acupuncture points can be identified as cutaneous neurogenic inflammatory spots. Sci Rep 7:15214
Sheng J, Jin X, Zhu J, Chen Y, Liu X (2019) The effectiveness of acupoint catgut embedding therapy for abdominal obesity: a systematic review and meta-analysis. Evid Based Complement Alternat Med 23:9714313
Kumar R, Mooventhan A, Manjunath NK (2017) Immediate effect of needling at CV-12 ,Zhongwan,acupuncture point on blood glucose level in patients with type 2 diabetes mellitus: a pilot randomized placebo-controlled trial. J Acupunct Meridian Stud 10:240–244
Yin J, Kuang J, Chandalia M, Tuvdendorj D, Tumurbaatar B, Abate N et al (2013) Hypoglycemic effects and mechanisms of electroacupuncture on insulin resistance. Am J Physiol Regul Integr Comp Physiol 307:R332–R339
Firouzjaei A, Li GC, Wang N, Liu WX, Zhu BM (2016) Comparative evaluation of the therapeutic effect of metformin monotherapy with metformin and acupuncture combined therapy on weight loss and insulin sensitivity in diabetic patients. Nutr Diabetes 6:e209
Darbandi M, Darbandi S, Owji AA, Mokarram P, Mobarhan MG, Fardaei M et al (2014) Auricular or body acupuncture: which one is more effective in reducing abdominal fat mass in Iranian men with obesity: a randomized clinical trial. J Diabetes Metab Disord 13:92
Sebayang RG, Aditya C, Abdurrohim K, Lauwrence B, Mihardja H, Kresnawan T et al (2020) Effects of laser acupuncture and dietary intervention on key obesity parameters. Med Acupunct 32:108–115
Yu Z, Xia Y, Ju C, Shao Q, Mao Z, Gu Y et al (2013) Electroacupuncture regulates glucose-inhibited neurons in treatment of simple obesity. Neural Regen Res 8:809–816
Saade MB, Cahu A, Moriez R, Neunlist M, Blat S (2019) Diet-induced obesity in young mice: consequences on the pancreatic intrinsic nervous system control of insulin secretion. Endocrinol Diabetes Metab 19:e00095
Li W, Yu G, Liu Y, Sha L (2019) Intrapancreatic ganglia and neural regulation of pancreatic endocrine secretion. Front Neurosci 13:21
Liang F, Koya D (2010) Acupuncture: is it effective for treatment of insulin resistance?[J]. Diabetes Obesity Metab 12:555–569
Liu S, Wang ZF, Su YS, Ray RS, Jing XH, Wang YQ et al (2020) somatotopic organization and intensity dependence in driving distinct NPY-expressing sympathetic pathways by electroacupuncture. Neuron 108:436–450.e7
Zhao C, Yang X, Su EM, Huang Y, Li L, Matthay MA et al (2017) Signals of vagal circuits engaging with AKT1 in α7 nAChR+CD11b+ cells lessen E. coli and LPS-induced acute inflammatory injury. Cell Discov 3:17009. https://doi.org/10.1038/celldisc.2017.9
Quinson N, Robbins HL, Clark MJ, Furness JB (2001) Locations and innervation of cell bodies of sympathetic neurons projecting to the gastrointestinal tract in the rat. Arch Histol Cytol 64:281–294
Jänig W, Morrison JF (1986) Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res 67:87–114
Gao X, Zhao Y, Su Y, Liu K, Yu X, Cui C et al (2016) β1/2 or M2/3 receptors are required for different gastrointestinal motility responses induced by acupuncture at heterotopic or homotopic acupoints. PLoS One 11:e0168200
Jänig W, Morrison JF (1986) Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res 67:87–114
Bear MF, Connors B, Paradiso M (2015) Neuroscience: Exploring the Brain, 3rd, edition. Lippincott Williams & Wilkins, New York, p. 493
Xue QM, Pan H, Huang L, Li N (2015) Effects of acupuncture at ST25 on inflammatory mediators and nuclear factor κB activation in a rat model of severe acute pancreatitis. Acupunct Med 33:299–304
Guo H, Zhu SF, Zhang RR, Zhao XL, Wan MH, Tang WF (2014) Electroacupuncture ameliorates acute lung injury through promoting gastrointestinal motility in rats with acute pancreatitis. Evid Based Complement Alternat Med 2014:943596
Ishizawa R, Kim HK, Hotta N, Iwamoto GA, Mitchell JH, Smith SA et al (2021) TRPV1 ,transient receptor potential vanilloid 1,sensitization of skeletal muscle afferents in type 2 diabetic rats with hyperglycemia. Hypertension 77:1360–1371
Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 52:313–320
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Joy KL, Kuttan R (1999) Anti-diabetic activity of Picrorrhiza kurroa extract. J Ethnopharmacol 67:143–148
Kirchgessner AL, Gershon MD (1990) Innervation of the pancreas by neurons in the gut. J Neurosci 10:1626–1642
Zoucas E, Nilsson C, Alm P et al (1996) Selective microsurgical sympathetic denervation of the rat pancreas. European Surgical Res 28:367–373
Maynard R, Downes N (2019) Anatomy and Histology of the Laboratory Rat in Toxicology and Biomedical Research, 1st edn. Academic Press, Pittsburgh, pp. 178–179
Lu MJ, Yu Z, He Y, Yin Y, Xu B (2019) Electroacupuncture at ST36 modulates gastric motility via vagovagal and sympathetic reflexes in rats. World J Gastroenterol 25:2315–2326
Zhang S, Gong P, Zhang J, Mao X, Zhao Y, Wang H et al (2020) Specific frequency electroacupuncture stimulation transiently enhances the permeability of the blood-brain barrier and induces tight junction changes. Front Neurosci 14:582324
Radin MS (2014) Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med 29:388–394
Myers MG Jr, Leibel RL, Seeley RJ, Schwartz MW (2010) Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 21:643–651
Kreymann B, Williams G, Ghatei MA, Bloom SR (2019) Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 1987(2):1300–1304
Nakamura Y Biomarkers for immune checkpoint inhibitor-mediated tumor response and adverse events. Front Med, Lausanne 6:119
Zhao G, Dharmadhikari G, Maedler K, Meyer-Hermann M (2014) Possible role of interleukin-1β in type 2 diabetes onset and implications for anti-inflammatory therapy strategies. PLoS Comput Biol 10:e1003798
Lin CC, Bradstreet TR, Schwarzkopf EA, Sim J, Carrero JA, Chou C et al (2014) Bhlhe40 controls cytokine production by T cells and is essential for pathogenicity in autoimmune neuroinflammation. Nat Commun 5:3551
Brereton MF, Iberl M, Shimomura K, Zhang Q, Adriaenssens AE, Proks P et al (2014) Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat Commun 5:4639
Gram DX, Holst JJ, Szallasi A (2017) TRPV1: a potential therapeutic target in type 2 diabetes and comorbidities? Trends Mol Med 9:1002–1013
DeFronzo RA, Eldor R, Abdul-Ghani M (2013) Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 36(Suppl 2):S127–S138
Gasmi A, Peana M, Arshad M, Butnariu M, Menzel A, Bjørklund G (2021) Krebs cycle: activators, inhibitors and their roles in the modulation of carcinogenesis. Arch Toxicol 95:1161–1178
Salehi B, Cruz-Martins N, Butnariu M, Sarac I, Bagiu IC, Ezzat SM et al (2021) Hesperetin's health potential: moving from preclinical to clinical evidence and bioavailability issues, to upcoming strategies to overcome current limitations. Crit Rev Food Sci Nutr 25:1–16
Baker RG, Hayden MS, Ghosh S (2011) NF-κB, inflammation, and metabolic disease. Cell Metab 13:11–22
Deane AM, Chapman MJ, Fraser RJ, Burgstad CM, Besanko LK, Horowitz M (2009) The effect of exogenous glucagon-like peptide-1 on the glycaemic response to small intestinal nutrient in the critically ill: a randomised double-blind placebo-controlled cross over study. Crit Care 13:R67
Shcherbina L, Lindqvist A, Thorén Fischer AH, Ahlqvist E, Zhang E, Falkmer SE et al (2018) Intestinal CART is a regulator of GIP and GLP-1 secretion and expression. Mol Cell Endocrinol 476:8–16
Abels M, Riva M, Bennet H, Ahlqvist E, Dyachok O, Nagaraj V et al (2016) CART is overexpressed in human type 2 diabetic islets and inhibits glucagon secretion and increases insulin secretion. Diabetologia 59:1928–1937
Wierup N, Richards WG, Bannon AW, Kuhar MJ, Ahrén B, Sundler F (2005) CART knock out mice have impaired insulin secretion and glucose intolerance, altered beta cell morphology and increased body weight. Regul Pept 129:203–211
D'souza AM, Neumann UH, Glavas MM, Kieffer TJ (2017) The glucoregulatory actions of leptin. Mol Metab 6:1052–1065
Tian HH, Cao BY, Li R, Ma YJ, Hu XG, Jia N et al (2018) Effects of electroacupuncture stimulation at different spinal segmental levels in a rat model of diabetes mellitus. Acupunct Med 36:29–35
Liao H, Xi P, Chen Q, Yi L, Zhao Y (2007) Clinical study on acupuncture, moxibustion, acupuncture plus moxibustion at Weiwanxiashu ,EX-B3,for treatment of diabetes. Zhongguo Zhen Jiu 27:482–484 Chinese
Kazemi AH, Wang W, Wang Y, Khodaie F, Rezaeizadeh H (2019) Therapeutic effects of acupuncture on blood glucose level among patients with type-2 diabetes mellitus: a randomized clinical trial. J Traditional Chin Med Sci 6:101–107
Wu L, Chen X, Liu Y, Lan J, Wu C, Li Z et al (2019) Role of acupuncture in the treatment of insulin resistance: a systematic review and meta-analysis. Complement Ther Clin Pract 37:11–22
Chen C, Liu J, Sun M, Liu W, Han J, Wang H (2019) Acupuncture for type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract 36:100–112
Jiang YL, Ning Y, Liu YY, Wang Y, Zhang Z, Yin LM et al (2011) Effects of preventive acupuncture on streptozotocin-induced hyperglycemia in rats. J Endocrinol Invest 34:e355–e361
Shou Y, Hu L, Zhang W, Gao Y, Xu P, Zhang B (2019) Determination of electroacupuncture effects on circRNAs in plasma exosomes in diabetic mice: an RNA-sequencing approach. Evid Based Complement Alternat Med 24:7543049
Kitahara A, Takahashi K, Morita N, Murashima T, Onuma H, Sumitani Y et al (2017) The novel mechanisms concerning the inhibitions of palmitate-induced proinflammatory factor releases and endogenous cellular stress with astaxanthin on MIN6 β-cells. Mar Drugs 15:185
Javed Z, Sadia H, Iqbal MJ, Shamas S, Malik K, Ahmed R et al (2021) Apigenin role as cell-signaling pathways modulator: implications in cancer prevention and treatment. Cancer Cell Int 21:189
Javed Z, Khan K, Herrera-Bravo J, Naeem S, Iqbal MJ, Sadia H et al (2021) Genistein as a regulator of signaling pathways and microRNAs in different types of cancers. Cancer Cell Int 21:388
Tracey KJ (2007) Physiology and immunology of the cholinergic anti-inflammatory pathway. J. Clin. Investig 117:289–296
Wang C, Cai X, Hu W, Li Z, Kong F, Chen X et al (2019) Investigation of the neuroprotective effects of crocin via antioxidant activities in HT22 cells and in mice with Alzheimer's disease. Int J Mol Med 43:956–966
Suri A, Szallasi A (2008) The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci 29:29–36. https://doi.org/10.1016/j.tips.2007.10.016
Lam D, Momeni Z, Theaker M, Jagadeeshan S, Yamamoto Y, Ianowski JP et al (2018) RAGE-dependent potentiation of TRPV1 currents in sensory neurons exposed to high glucose. PLoS One 13:e0193312
Zhang N, Gao D, Liu Y, Ji S, Sha L (2018) Effects of neuropeptide substance P on proliferation and β-cell differentiation of adult pancreatic ductal cells. Front Neurosci 12:806
Dirice E, De Jesus DF, Kahraman S, Basile G, Ng RW et al (2019) Human duct cells contribute to β cell compensation in insulin resistance. JCI Insight 4:e99576
Lázár BA, Jancsó G, Sántha P (2020) Modulation of sensory nerve function by insulin: possible relevance to pain, inflammation and axon growth. Int J Mol Sci 21:2507
Hermansen K, Ahrén B (1990) Dual effects of calcitonin gene-related peptide on insulin secretion in the perfused dog pancreas. Regul Pept 27:149–157
Sanford D, Luong L, Gabalski A, Oh S, Vu JP, Pisegna JR et al (2019) An intraperitoneal treatment with calcitonin gene-related peptide ,CGRP,regulates appetite, energy intake/expenditure, and metabolism. J Mol Neurosci 67:28–37
Pettersson M, Ahrén B, Böttcher G, Sundler F (1986) Calcitonin gene-related peptide: occurrence in pancreatic islets in the mouse and the rat and inhibition of insulin secretion in the mouse. Endocrinology 119:865–869
Bou Karam J, Cai W, Mohamed R, Huang T, Meng L, Homan EP et al (2018) TRPV1 neurons regulate β-cell function in a sex-dependent manner. Mol Metab 18:60–67
Sonne DP, Hemmingsen B (2017) Comment on American Diabetes Association. Standards of Medical Care in Diabetes-2017. Diabetes Care 40:e92–e93
Lamos EL, Stein SA, Davis SN (2013) Sulfonylureas and meglitinides: historical and contemporary issues. Panminerva Med 55:239–251
Pendharkar SA, Walia M, Drury M, Petrov MS (2017) Calcitonin gene-related peptide: neuroendocrine communication between the pancreas, gut, and brain in regulation of blood glucose. Ann Transl Med 5:419
Mayer F, Gunawan AL, Tso P, Aponte GW (2020) Glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide stimulate release of substance P from TRPV1- and TRPA1-expressing sensory nerves. Am J Physiol Gastrointest Liver Physiol 319:G23–G35
Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J et al (2006) TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell 127:1123–1135
Acknowledgements
We are grateful to the staff of the Key Laboratory of Acupuncture and Medicine Research of the Ministry of Education in the Nanjing University of Chinese Medicine for their support during the preparation of this manuscript.
Availability of Data and Materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported in part by the National Natural Science Foundation of China (grant numbers 81873238, 81873371, 82074532, 81673883 and 81574171, National Key R&D Program of China (grant number 2020YFC2006600), the Provincial Natural Science Foundation of Jiangsu (grant number BK20181420), the Postgraduate Research Innovation Program of Jiangsu Province (grant number KYCX_1472 and KYCX_1696), the Leading Talents of Traditional Chinese Medicine in Jiangsu (grant number SLJ0225), and the Research Project of Nanjing University of Chinese Medicine (grant number XT202001).
Author information
Authors and Affiliations
Contributions
Conceptualization: Tiancheng Xu, Zhi Yu, and Yun Liu; methodology and software and visualization: Tiancheng Xu and Mengjiang Lu; data curation and supervision: Zhi Yu, Meirong Gong, and Qian Li; writing—original draft preparation: Tiancheng Xu and Yun Liu; writing—review and editing: Zhi Yu; project administration and funding acquisition: Youbing Xia and Bin Xu; all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experimental procedures were conducted according to the guidelines for animal experimentation of the Nanjing University of Chinese Medicine and were approved by the Ethics Committee for Animal Experimentation (under grant 202006A016).
Consent to Participate
Informed consent was obtained from the animal research center of the Nanjing University of Chinese Medicine (No. 1100112011052760, under grant SCXK(JING)2016-0006).
Consent for Publication
Written informed consent for publication was obtained from all participants.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, T., Yu, Z., Liu, Y. et al. Hypoglycemic Effect of Electroacupuncture at ST25 Through Neural Regulation of the Pancreatic Intrinsic Nervous System. Mol Neurobiol 59, 703–716 (2022). https://doi.org/10.1007/s12035-021-02609-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02609-1