Abstract
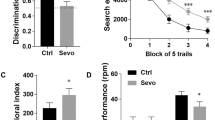
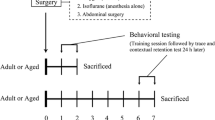
Neuroinflammation can cause cognitive deficits, and preexisting neuroinflammation is observed frequently in the clinic after trauma, surgery, and infection. Patients with preexisting neuroinflammation often need further medical treatment under general anesthesia. However, the effects of postconditioning with general anesthetics on preexisting neuroinflammation have not been determined. In this study, adult rats were posttreated with sevoflurane or propofol after intracerebroventricular administration of lipopolysaccharide. The effects of sevoflurane or propofol postconditioning on neuroinflammation-induced recognition memory deficits were detected. Our results found that postconditioning with sevoflurane but not propofol reversed the selective spatial recognition memory impairment induced by neuroinflammation, and these differential effects did not appear to be associated with the similar anti-neuroinflammatory responses of general anesthetics. However, postconditioning with propofol induced a selective long-lasting upregulation of extrasynaptic NR2B-containing N-methyl-D-aspartate receptors in the dorsal hippocampus, which downregulated the cAMP response element-binding signaling pathway and impaired spatial recognition memory. Additionally, the NR2B antagonists memantine and Ro25-6981 reversed this neurotoxicity induced by propofol postconditioning. Taken together, these results indicate that under preexisting neuroinflammation, postconditioning with sevoflurane can provide reliable neuroprotection by attenuating lipopolysaccharide-induced neuroinflammation, apoptosis, and neuronal loss and eventually improving spatial recognition deficits. However, although posttreatment with propofol also has the same anti-neuroinflammatory effects, the neurotoxicity caused by propofol postconditioning following neuroinflammation warrants further consideration.






Similar content being viewed by others
Data Availability
The data used during the present study are available from the corresponding author upon reasonable request.
References
Kitano H, Kirsch JR, Hurn PD, Murphy SJ (2007) Inhalational anaesthetics as neuroprotectants or chemical1preconditioning agents in ischemic brain. J Cereb Blood Flow Metab 27(6):1108–1128. https://doi.org/10.1038/sj.jcbfm.9600410
Wang J-K, Yu L-N, Zhang F-J, Yang M-J, Yu J, Yan M et al (2010) Postconditioning with sevoflurane protects against focal cerebral ischemia and reperfusion injury via PI3K/Akt pathway. Brain Res 1357:142–151. https://doi.org/10.1016/j.brainres.2010.08.009
Hwang J-W, Jeon Y-T, Lim Y-J, Park H-P (2017) Sevoflurane postconditioning-induced anti-inflammation via inhibition of the toll-like receptor-4/nuclear factor kappa B pathway contributes to neuroprotection against transient global cerebral ischemia in rats. Int J Mol Sci 18(11):2347. https://doi.org/10.3390/ijms18112347
Wang H, Wang G, Yu Y, Wang Y (2009) The role of phosphoinositide-3-kinase/Akt pathway in4. propofol-induced postconditioning against focal cerebral ischemia-reperfusion injury in rats. Brain Res 1297:177–184. https://doi.org/10.1016/j.brainres.2009.08.054
Liang C, Cang J, Wang H, Xue Z (2013) Propofol attenuates cerebral ischemia/reperfusion injury partially using heme oxygenase-1. J Neurosurg Anesthesiol 25(3):311–316. https://doi.org/10.1097/ANA.0b013e31828c6af5
Huang C, Ng OT-W, Chu JM-T, Irwin MG, Hu X, Zhu S et al (2019) Differential effects of propofol and6. dexmedetomidine on neuroinflammation induced by systemic endotoxin lipopolysaccharides in adult mice. Neurosci Lett 707:134309. https://doi.org/10.1016/j.neulet.2019.134309
Ye X, Lian Q, Eckenhoff MF, Eckenhoff RG, Pan JZ (2013) Differential general anesthetic effects on microglial cyktokine expression. PLoS One 8(1):e52887. https://doi.org/10.1371/journal.pone.0052887
Shaw K, Commins S, O’Mara SM (2001) Lipopolysaccharide causes deficits in spatial learning in the water maze but not in BDNF expression in the rat dentate gyrus. Behav Brain Res 124(1):47–54. https://doi.org/10.1016/s0166-4328(01)00232-7
Sparkman NL, Kohman RA, Scott VJ, Boehm GW (2005) Bacterial endotoxin-induced behavioral alterations in two variations of the Morris water maze. Physiol Behav 86(1-2):244–251. https://doi.org/10.1016/j.physbeh.2005.07.016
Irifune M, Takarada T, Shimizu Y, Endo C, Katayama S, Dohi T et al (2003) Propofol-induced anaesthesia in mice is mediated by gamma-aminobutyric acid-A and excitatory amino acid receptors. Anesth Analg 97(2):424–429. https://doi.org/10.1213/01.ane.0000059742.62646.40
Kelly EW, Solt K, Raines DE (2007) Volatile aromatic anaesthetics variably impact gamma-aminobutyric acid type A receptor function. Anesth Analg 105(5):1287–1292. https://doi.org/10.1213/01.ane.0000282829.21797.97
Newcomer JW, Krystal JH (2001) NMDA receptor regulation of memory and behavior in humans. Hippocampus 11(5):529–542. https://doi.org/10.1002/hipo.1069
Furukawa H, Singh SK, Mancusso R, Gouaux E (2005) Subunit arrangement and function in NMDA receptor. Nature 438(7065):185–192. https://doi.org/10.1038/nature04089
Rammes G, Starker LK, Haseneder R, Berkmann J, Alexandra P, Zieglgänsberger W (2009) Isoflurane anaesthesia reversibly improve cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology 56(3):626–636. https://doi.org/10.1016/j.neuropharm.2008.11.002
Monaco SA, Gulchina Y, Gao W-J (2015) NR2B subunit in the prefrontal cortex: A double-edged sword for working memory function and psychiatric disorders. Neurosci Biobehav Rev 56:127–138. https://doi.org/10.1016/j.neubiorev.2015.06.022
Liu J, Zhang X, Zhang W, Gu G, Wang P (2015) Effects of sevoflurane on young male adult C57BL/6 mice spatial cognition. PLoS One 10(8):e0134217. https://doi.org/10.1371/journal.pone.0134217
Wang Y, Han S, Han R, Su Y, Li J (2017) Propofol-induced downregulation of NR2B membrane translacation in hippocampus and spatial memory deficits of neonatal mice. Brain Behav 7(7):e00734. https://doi.org/10.1002/brb3.734
Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE et al (2009) Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med 15(12):1407–1413. https://doi.org/10.1038/nm.2056
Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ (2011) Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci 31(18):6627–6638. https://doi.org/10.1523/JNEUROSCI.0203-11.2011
Hardingham GE, Bading H (2010) Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11(10):682–696. https://doi.org/10.1038/nrn.2911
Hardingham GE, Fukunaga Y, Bading H (2002) Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5(5):405–414. https://doi.org/10.1038/nn835
Gouix E, Léveillé F, Nicole O, Melon C, Had-Aissouni L, Buisson A (2009) Reverse glial glutamate uptake triggers neuronal cell death through extrasynaptic NMDA receptor activation. Mol Cell Neurosci 40(4):463–473. https://doi.org/10.1016/j.mcn.2009.01.002
Wang W-Y, Jia L-J, Luo Y, Zhang H-H, Cai F, Mao H et al (2016) Location- and subunit-specific NMDA receptors determine the developmental sevoflurane neurotoxicity through ERK1/2 signaling. Mol Neurobiol 53(1):216–230. https://doi.org/10.1007/s12035-014-9005-1
Li L, Li Z, Cao Y, Fan D, Chui D, Guo X (2016) Increased extrasynaptic GluN2B expression is involved in cognitive impairment after isoflurane anesthesia. Exp Ther Med 12(1):161–168. https://doi.org/10.3892/etm.2016.3306
Gong Q-H, Wang Q, Pan L-L, Liu X-H, Huang H, Zhu Y-Z (2010) Hydrogen sulfide attenuates lipopolysaccharide-induecd cognitive impairment: a proinflammatory pathway in rats. Pharmacol Biochem Behav 96(1):52–58. https://doi.org/10.1016/j.pbb.2010.04.006
Vedder LC, Smith CC, Flannigan AE, McMahon LL (2013) Estradiol-induced increase in novel object recognition requires hippocampal NR2B-containing NMDA receptors. Hippocampus 23(1):108–115. https://doi.org/10.1002/hipo.22068
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Elesvier
Jiang CH, Lee S, Park IY, Song A, Moon C, Cho GW (2019) Memantine attenuates salicylate-induced tinnitus possibly by reducing NR2B expression in auditory cortex of rat. Exp Neurobiol 28(4):495–503. https://doi.org/10.5607/en.2019.28.4.495
Barker GR, Warburton EC (2011) When is the hippocampus involved in recognition memory? J Neurosci 31(29):10721–10731. https://doi.org/10.1523/JNEUROSCI.6413-10.2011
Chen B, Deng X, Wang B, Liu H (2016) Etanercept, an inhibitor of TNF-α, prevents propofol-induced neurotoxicity in the developing brain. Int J Dev Neurosci 55:91–100. https://doi.org/10.1016/j.ijdevneu.2016.10.002
Chen B, Deng X, Wang B, Liu H (2016) Persistent neuronal apoptosis and synaptic loss induced by multiple but not single exposure of propofol contribute to long-term cognitive dysfunction in neonatal rats. J Toxicol Sci 41(5):627–636. https://doi.org/10.2131/jts.41.627
Zhang Z, Song Z, Shen F, Xie P, Wang J, Zhu A, Zhu G (2020) Ginsenoside Rg1 prevents PTSD-like behaviors in mice through promoting synaptic proteins, reducing Kir4.1 and TNF-α in the hippocampus. Mol Neurobiol. https://doi.org/10.1007/s12035-020-02213-9
Huttner WB, Schiebler W, Greengard P, De Camilli P (1983) Synapsin-I (Protein-I), a nerve terminal-specific phosphoprotein .III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol 96(5):1374–1388. https://doi.org/10.1083/jcb.96.5.1374
Carlin RK, Grab DJ, Cohen RS, Siekevitz P (1980) Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol 86(3):831–845. https://doi.org/10.1083/jcb.86.3.831
Smith KE, Gibson ES, Dell’Acqua ML (2006) cAMP-dependent protein kinase postsynaptic localization regulated by NMDA receptor activation through translocation of an A-kinase anchoring protein scaffold protein. J Neurosci 26(9):2391–2402. https://doi.org/10.1523/JNEUROSCI.3092-05.2006
Matus AI, Taff-Jones DH (1978) Morphology and molecular composition of isolated postsynaptic junctional structures. Proc R Soc Lond B Biol Sci 203(1151):135–151. https://doi.org/10.1098/rspb.1978.0097
Philips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W et al (2001) The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron 32(1):63–77. https://doi.org/10.1016/s0896-6273(01)00450-0
Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD et al (2010) Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron 65(2):178–190. https://doi.org/10.1016/j.neuron.2010.01.008
Deng X, Chen B, Wang B, Zhang J, Liu H (2017) TNF-α mediates the intrinsic and extrinsic pathway in propofol-induced neuronal apoptosis via PI3K/Akt signaling pathway in rat prefrontal cortical neurons. Neurotox Res 32(3):409–419. https://doi.org/10.1007/s12640-017-9751-8
Shi D-D, Huang Y-H, Lai CSW, Dong CM, Ho LC, Li X-Y et al (2019) Ginsenoside Rg1 prevents chemotherapy-induced cognitive impairment: associations with microglia-mediated cytokines, neuroinflammation, and neuroplasticity. Mol Neurobiol 56:5626–5642. https://doi.org/10.1007/s12035-019-1474-9
Zarifkar A, Choopani S, Ghasemi R, Naghdi N, Maghsoudi AH, Maghsoudi N et al (2010) Agmatine prevents LPS-induced spatial memory impairment and hippocampal apoptosis. Eur J Pharmacol 634(1-3):84–88. https://doi.org/10.1016/j.ejphar.2010.02.029
Feng Y, Xue H, Zhu J, Yang L, Zhang F, Qian R et al (2016) ESE1 is associated with neuronal apoptosis in lipopolysaccharide induced neuroinflammation. Neurochem Res 41(10):2752–2762. https://doi.org/10.1007/s11064-016-1990-1
Wang K-C, Fan L-W, Kaizaki A, Pang Y, Cai Z, Tien L-T (2013) Neonatal lipopolysaccharide exposure induces long-lasting learning impairment, less anxiety-like response and hippocampal injury in adult rats. Neuroscience 234:146–157. https://doi.org/10.1016/j.neuroscience.2012.12.049
Lu W, Roche KW (2012) Posttranslational regulation of AMPA receptor trafficking and function. Curr Opin Neurobiol 22(3):470–479. https://doi.org/10.1016/j.conb.2011.09.008
Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12(3):529–540. https://doi.org/10.1016/0896-6273(94)90210-0
Parsons MP, Raymond LA (2014) Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 82(2):279–293. https://doi.org/10.1016/j.neuron.2014.03.030
Varbanov H, Dityatev A (2017) Regulation of extrasynaptic signaling by polysialylated NCAM: impact for synaptic plasticity and cognitive function. Mol Cell Neurosci 81:12–21. https://doi.org/10.1016/j.mcn.2016.11.005
Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD (2009) Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience 158(4):1446–1459. https://doi.org/10.1016/j.neuroscience.2008.11.006
Stazi M, Wirths O (2021) Chronic memantine treatment ameliorates behavioral deficits, neuron loss, and impaired neurogenesis in a model of Alzheimer’s disease. Mol Neurobiol 58(1):204–216. https://doi.org/10.1007/s12035-020-02120-z
Fisher G, Mutel V, Trube G, Malherbe P, Kew JN, Mohasci E et al (1997) Ro25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther 283(3):1285–1292
Zarbato GF, de Souza Goldim MP, Giustina AD, Danielski LG, Mathias K, Florentino D et al (2018) Dimethyl fumarate limits neuroinflammation and oxidative stress and improves cognitive impairment after polymicrobial sepsis. Neurotox Res 34(3):418–430. https://doi.org/10.1007/s12640-018-9900-8
Safavynia SA, Goldstein PA (2019) The role of neuroinflammation in postoperative cognitive dysfunction: moving from hypothesis to treatment. Front Psychiatry 9:752. https://doi.org/10.3389/fpsyt.2018.00752
Tripathi A, Paliwal P, Krishnamurthy S (2017) Piracetam attenuates LPS-induced neuroinflammation and cognitive impairment in rats. Cell Mol Neurobiol 37(8):1373–1386. https://doi.org/10.1007/s10571-017-0468-2
Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH et al (2012) TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation 9:23. https://doi.org/10.1186/1742-2094-9-23
Cholvin T, Loureiro M, Cassel R, Cosquer B, Herbeaux K, de Vasconcelos AP et al (2016) Dorsal hippocampus and medial prefrontal cortex each contribute to the retrieval of a recent spatial memory in rats. Brain Struct Funct 221(1):91–102. https://doi.org/10.1007/s00429-014-0894-6
Ennaceur A, Neave N, Aggleton JP (1996) Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav Brain Res 80(1-2):9–25. https://doi.org/10.1016/0166-4328(96)00006-x
Hannesson DK, Vacca G, Howland JG, Phillips AG (2004) Medial prefrontal cortex is involved in spatial temporal order memory but not spatial recognition memory in tests relying on spontaneous exploration in cortex. Psychopharmacology 232(24):4433–4444. https://doi.org/10.1007/s00213-015-4071-2
Tosaka S, Tosaka R, Matsumoto S, Maekawa T, Cho S, Sumikawa K (2011) Roles of cyclooxygenase 2 in sevoflurane- and olprinone-induced early phase of preconditioning and postconditioning against myocardial infarction in rat hearts. J Cardiovasc Pharmacol Ther 16(1):72–78. https://doi.org/10.1177/1074248410380208
Hu X, Wang J, Zhang Q, Duan X, Chen Z, Zhang Y (2016) Postconditioning with sevoflurane amileorates spatial learning and memory deficit after hemorrhage shock and resuscitation in rats. J Surg Res 206(2):307–315. https://doi.org/10.1016/j.jss.2016.08.026
Luo C, Zhang YL, Luo W, Zhou FH, Li CQ, Xu JM, et al (2015) Differential effects of general anesthetics on anxiety-like behavior in formalin-induced pain: involvement of ERK activation in the anterior cingulate
Schoen J, Husemann L, Tiemeyer C, Lueloh A, Sedemund-Adib B, Berger KU et al (2011) Cognitive function after sevoflurane- vs propofol-based anaesthesia for on-pump cardiac surgery: a randomized controlled trial. Br J Anaesth 106(6):840–850. https://doi.org/10.1093/bja/aer091
Hardingham GE, Bading H (2003) The yin and yang of NMDA receptor signalling. Trends Neurosci 26(2):81–89. https://doi.org/10.1016/S0166-2236(02)00040-1
Warburton EC, Barker GR, Brown MW (2013) Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology 74(100):41–47. https://doi.org/10.1016/j.neuropharm.2013.04.013
Réus GZ, Valvassori SS, Machado RA, Martins MR, Gavioli EC, Quevedo J (2007) Acute treatment with low doses of memantine does not impair aversive, non-associative and recognition memory in rats. Naunyn Schmiedeberg's Arch Pharmacol 376(5):295–300. https://doi.org/10.1007/s00210-007-0235-x
Danysz W, Parsons CG (2002) Neuroprotective potential of ionotropic glutamate receptor antagonists. Neurotox Res 4(2):119–126. https://doi.org/10.1080/10298420290015872
Wu Q, Zheng R, Srisai D, McKnight GS, Palmiter RD (2013) NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus. Proc Natl Acad Sci U S A 110(36):14765–14770. https://doi.org/10.1073/pnas.1314137110
Jiang M, Zhang W, Ma Z, Gu X (2013) Antinociception and prevention of hyperalgesia by intrathecal administration of Ro 25-6981, a highly selective antagonist of the 2B subunit of N-methyl-D-aspartate receptor. Pharmacol Biochem Behav 112:56–63. https://doi.org/10.1016/j.pbb.2013.09.007
Zhan Y, Xia J, Wang X (2020) Effects of glutamate-related drugs on anxiety and compulsive behavior in rats with obsessive-compulsive disorder. Int J Neurosci 130(6):551–560. https://doi.org/10.1080/00207454.2019.1684276
Chen Y, Chen A, Luo X, Guo L, Tang Y, Bao C et al (2014) Hippocampal NR2B-containing NMDA receptors enhance long-term potentiation in rats with chronic visceral pain. Brain Res 1570:43–53. https://doi.org/10.1016/j.brainres.2014.05.001
Luciano-Jaramillo J, Sandoval-García F, Vázquez-Del Mercado M, Gutiérrez-Mercado YK, Navarro-Hernández RE, Martínez-García EA et al (2019) Downregulation of hippocampal NR2A/2B subunits related to cognitive impairment in a pristane-induced lupus BALB/c mice. PLoS One 14(9):e0217190. https://doi.org/10.1371/journal.pone.0217190
Chen D, Qi X, Zhuang R, Cao J, Xu Y, Huang X et al (2019) Prenatal propofol exposure downregulates NMDA receptor expression and causes cognitive and emotional disorders in rats. Eur J Pharmacol 843:268–276. https://doi.org/10.1016/j.ejphar.2018.11.032
Stabernack C, Sonner JM, Laster M, Zhang Y, Xing Y, Sharma M et al (2003) Spinal N-methyl-d-aspartate receptors may contribute to the immobilizing action of isoflurane. Anesth Analg 96(1):102–107. https://doi.org/10.1097/00000539-200301000-00022
Franks NP (2006) Molecular targets underlying general anaesthesia. Br J Pharmacol 147(Suppl 1(Suppl1)):S72–S81. https://doi.org/10.1038/sj.bjp.0706441
Gladding CM, Raymond LA (2011) Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol Cell Neurosci 48(4):308–320. https://doi.org/10.1016/j.mcn.2011.05.001
Wang WY, Luo Y, Jia LJ, Hu SF, Lou XK, Shen SL et al (2014) Inhibition of aberrant cyclin-dependent kinase 5 activity attenuates isoflurane neurotoxicity in the developing brain. Neuropharmacology 77:90–99. https://doi.org/10.1016/j.neuropharm.2013.09.006
Yang X, Zhang W, Wu H, Fu S, Yang J, Liu S et al (2020) Downregulation of CDK5 restores sevoflurane-induced cognitive dysfunction by promoting SIRT1-mediated autophagy. Cell Mol Neurobiol 40(6):955–965. https://doi.org/10.1007/s10571-020-00786-6
Zhang S, Edelmann L, Liu J, Crandall JE, Morabito MA (2008) Cdk5 regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors. J Neurosci 28(2):415–424. https://doi.org/10.1523/JNEUROSCI.1900-07.2008
Alves HN, da Silva AL, Olsson IA, Orden JM, Antunes LM (2010) Anesthesia with intraperitoneal propofol, medetomidine, and fentanyl in rats. J Am Assoc Lab Anim Sci 49(4):454–459
Dantzer R (2001) Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci 933:222–234. https://doi.org/10.1111/j.1749-6632.2001.tb05827.x
Steffey MA, Brosnan RJ, Steffey EP (2003) Assessment of halothane and sevoflurane anesthesia in spontaneously breathing rats. Am J Vet Res 64(4):470–474. https://doi.org/10.2460/ajvr.2003.64.470
Acknowledgements
We wish to thank Lixue Chen, Guangcheng Qing, and Dunke Zhang for their excellent technical assistance.
Funding
This study was supported by grants from the National Science Foundation Project of Chongqing (cstc2017jcyjBX0043 and cstc2019jcyj-msxmX0608).
Author information
Authors and Affiliations
Contributions
Bo Chen: conceptualization, methodology, formal analysis, investigation, writing–original draft, funding acquisition. Bianqin Guo: methodology, investigation. Xiaoyuan Deng: formal analysis, investigation, writing–original draft. Bin Wang: methodology, investigation, writing–review and editing. Xiaoyun Dou: investigation. Hongliang Liu: conceptualization, methodology, writing–original draft, writing–review and editing, funding acquisition.
Corresponding author
Ethics declarations
This study was conducted according to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH) and the approval of the Committee on the Ethics of Animal Experiments of Chongqing University Cancer Hospital (Chongqing, P.R. China: Approval No. SCXK 2018-0003).
Conflict of Interest
The authors declare no competing interests.
Consent to Participate
Not applicable.
Consent for Publication
All authors consent to the publication of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bo Chen and Bianqin Guo are the co-first authors to this work.
Rights and permissions
About this article
Cite this article
Liu, H., Chen, B., Guo, B. et al. Postconditioning with Sevoflurane or Propofol Alleviates Lipopolysaccharide-Induced Neuroinflammation but Exerts Dissimilar Effects on the NR2B Subunit and Cognition. Mol Neurobiol 58, 4251–4267 (2021). https://doi.org/10.1007/s12035-021-02402-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02402-0