Abstract
In peripheral neuropathies, axonal degeneration (AxD) impairs the prognosis for recovery. Here, we describe a role for dual specificity phosphatases (DUSPs; MAP kinase phosphatases, MKPs), in supporting autonomous axon plasticity and viability. Both DUSPs 1 and 4 were identified within intact or axotomized sensory neurons. Knockdown of DUSP 1 or 4 independently or combined impaired neurite outgrowth in adult dissociated sensory neurons. Furthermore, adult sensory neurons with DUSP knockdown were rendered sensitive to axonopathy in vitro following exposure to low, subtoxic TrpV1 (transient receptor potential cation channel subfamily V member 1) activation by capsaicin, an intervention normally supportive of growth. This was not prevented by concurrent DLK (dual leucine zipper kinase) knockdown. Ex vivo neurofilament dissolution was heightened by DUSP inhibition within explanted nerves. In vivo DUSP knockdown or inhibition was associated with more rapid loss of motor axon excitability. The addition of SARM1 (sterile alpha and TIR motif containing 1) siRNA abrogated DUSP1 and 4 mediated loss of excitability. DUSP knockdown accelerated neurofilament breakdown and there was earlier morphological evidence of myelinated axon degeneration distal to axotomy. Taken together, the findings identify a key role for DUSPs in supporting axon plasticity and survival.








Similar content being viewed by others
References
Gerdts J, Summers DW, Milbrandt J, Di Antonio A (2016) Axon self-destruction: new links among SARM1, MAPKs, and NAD+ metabolism. Neuron 89:449–460
Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J (2015) SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science 348:453–457
Sasaki Y, Vohra BP, Baloh RH, Milbrandt J (2009) Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J Neurosci 29:6526–6534
Sasaki Y, Vohra BP, Lund FE, Milbrandt J (2009) Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci 29:5525–5535
Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, Di Antonio A (2009) A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci 12:387–389
Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T et al (2001) Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci 4:1199–1206
Wang MS, Fang G, Culver DG, Davis AA, Rich MM, Glass JD (2001) The WldS protein protects against axonal degeneration: a model of gene therapy for peripheral neuropathy. Ann Neurol 50:773–779
Zochodne DW (2008) Neurobiology of peripheral nerve regeneration. Cambridge Press, Cambridge
Rios P, Nunes-Xavier CE, Tabernero L, Kohn M, Pulido R (2014) Dual-specificity phosphatases as molecular targets for inhibition in human disease. Antioxid Redox Signal 20:2251–2273
Patterson KI, Brummer T, O'Brien PM, Daly RJ (2009) Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J 418:475–489
Cheng C, Kobayashi M, Martinez JA, Ng H, Moser JJ, Wang X, Singh V, Fritzler MJ et al (2015) Evidence for epigenetic regulation of gene expression and function in chronic experimental diabetic neuropathy. J Neuropathol Exp Neurol 74:804–817
Walker LJ, Summers DW, Sasaki Y, Brace EJ, Milbrandt J, DiAntonio A (2017) MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. Elife 6:e22540 [doi: 10.7554]
Christie KJ, Webber CA, Martinez JA, Singh B, Zochodne DW (2010) PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J Neurosci 30:9306–9315
Cheng C, Guo GF, Martinez JA, Singh V, Zochodne DW (2010) Dynamic plasticity of axons within a cutaneous milieu. J Neurosci 30:14735–13744
Christie KJ, Krishnan A, Martinez JA, Purdy K, Singh B, Eaton S, Zochodne D (2014) Enhancing adult nerve regeneration through the knockdown of retinoblastoma protein. Nat Commun 5:3670 [doi: 10.1038]
Woo V, Cheng C, Duraikannu A, Chandrasekhar A, Purdy K, Martinez JA, Zochodne DW (2018) Caspase-6 is a dispensable enabler of adult mammalian axonal degeneration. Neuroscience 371:242–253
Poitras T, Chandrasekhar A, McCoy L, Komirishetty P, Areti A, Webber CA, Zochodne DW (2019) Selective sensory axon reinnervation and TRPV1 activation. Mol Neurobiol 56:7144–7158
Liu R, Molkentin JD (2016) Regulation of cardiac hypertrophy and remodeling through the dual-specificity MAPK phosphatases (DUSPs). J Mol Cell Cardiol 101:44–49
Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, Di Antonio A (2012) Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 74:1015–1022
Zigmond RE, Echevarria FD (2019) Macrophage biology in the peripheral nervous system after injury. Prog Neurobiol 173:102–121
Lindborg JA, Niemi JP, Howarth MA, Liu KW, Moore CZ, Mahajan D, Zigmond RE (2018) Molecular and cellular identification of the immune response in peripheral ganglia following nerve injury. J Neuroinflammation 15:192 [doi: 10.1186]
Wang J, Zhou JY, Kho D, Reiners JJ Jr, Wu GS (2016) Role for DUSP1 (dual-specificity protein phosphatase 1) in the regulation of autophagy. Autophagy 12:1791–1803
Koga S, Kojima S, Kishimoto T, Kuwabara S, Yamaguchi A (2012) Over-expression of map kinase phosphatase-1 (MKP-1) suppresses neuronal death through regulating JNK signaling in hypoxia/re-oxygenation. Brain Res 1436:137–146
Morente V, Perez-Sen R, Ortega F, Huerta-Cepas J, Delicado EG, Miras-Portugal MT (2014) Neuroprotection elicited by P2Y13 receptors against genotoxic stress by inducing DUSP2 expression and MAPK signaling recovery. Biochim Biophys Acta 1843:1886–1898
Queipo MJ, Gil-Redondo JC, Morente V, Ortega F, Miras-Portugal MT, Delicado EG, Perez-Sen R (2017) P2X7 nucleotide and EGF receptors exert dual modulation of the dual-specificity phosphatase 6 (MKP-3) in granule neurons and astrocytes, contributing to negative feedback on ERK signaling. Front Mol Neurosci 10:448 [doi: 10.3389]
Pastuhov SI, Hisamoto N, Matsumoto K (2015) MAP kinase cascades regulating axon regeneration in Celegans. Proc Jpn Acad Ser B Phys Biol Sci 91:63–75
Asghari AE, Smithson LJ, Collins CA (2018) An axonal stress response pathway: degenerative and regenerative signaling by DLK. Curr Opin Neurobiol 53:110–119
Tedeschi A, Bradke F (2013) The DLK signalling pathway--a double-edged sword in neural development and regeneration. EMBO Rep 14:605–614
Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M (2005) Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 45:715–726
Singh B, Krishnan A, Micu I, Koshy K, Singh V, Martinez JA, Koshy D, Xu F et al (2015) Peripheral neuron plasticity is enhanced by brief electrical stimulation and overrides attenuated regrowth in experimental diabetes. Neurobiol Dis 83:134–151
Mahar M, Cavalli V (2018) Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci 19:323–337
Senger JB, Verge VMK, Chan KM, Webber CA (2018) The nerve conditioning lesion: a strategy to enhance nerve regeneration. Ann Neurol 83:691–702
Frey E, Karney-Grobe S, Krolak T, Milbrandt J, Di Antonio A (2018) TRPV1 agonist, capsaicin, induces axon outgrowth after injury via Ca(2+)/PKA signaling. eNeuro. 5:3 [doi: 10.1523]
Acknowledgments
Twinkle Joy provided technical assistance.
Funding
The work was supported by an operating grant from the Canadian Institutes of Health Research (FRN148675).
Author information
Authors and Affiliations
Contributions
AC participated in initial drafts of the paper, set up and designed experiments, helped to supervise the overall project, collated and analyzed results, and contributed to the intellectual content of the work. PK, AA, and AK set up and designed experiments for the work, collated and analyzed results, and contributed to the intellectual content of the work. DZ supervised the project, directed its overall methodology, and generated drafts of the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
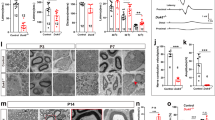
Supplemental Figure 1: (A,B) Longitudinal images of proximal stump of axotomized (3 days) mouse sciatic nerves colabelled with neurofilament (red) and either DUSP1 (green[A]) or DUSP4 green[B]) and DAPI (blue). The inset confirms axon double labeling (inset, yellow) for DUSP1 but not DUSP 4 (inset). The findings confirm DUSP1 axon localization in proximal stumps after axotomy whereas DUSP4 was expressed in periaxonal glial cells. Bar= 25 µM (PNG 6057 kb)
ESM 2
Supplemental Figure 2: (A,B) Longitudinal images of distal stumps of axotomized (3 days) mouse sciatic nerves colabelled with neurofilament (red in A, green in B) and either DUSP1 (green[A]) or DUSP4 (red [B]) and DAPI for nuclei (blue). The inset confirms axon double labeling (inset, yellow) for DUSP1 but not DUSP 4 (inset). The findings confirm DUSP1 axon localization in distal stumps after axotomy whereas DUSP4 was expressed in periaxonal glial cells. Bar= 25 µM (PNG 6628 kb)
ESM 3
Supplemental Figure 3: (A-C) DUSP1 siRNA knockdown had no impact on sensory neuron sprouting or branching. Comparable numbers of neurons were available for analysis in the scrambled compared to DUSP1 siRNA groups [n=5/group]. (D-F) DUSP4 siRNA knockdown was associated with reduced sprouting and branching. Comparable numbers of neurons were available for analysis in the scrambled compared to DUSP4 siRNA groups. Results from A-F are from the same experimental groups used to measure neurite outgrowth described in Figure 1. [#p=0.04, scrambled vs. DUSP4 siRNA, one tailed unpaired Student’s t-test, n=3/group; *p=0.0021, scrambled vs. DUSP4 siRNA, two tailed unpaired Student’s t-test, n=3/group] (PNG 360 kb)
ESM 4
Supplemental Figure 4: (A-C) DLK siRNA knockdown was associated with reduced sprouting (A) and reduced branching (B). Comparable numbers of neurons were available for analysis in the scrambled compared to DUSP4 siRNA groups (C). Results from A-C are from the same experimental groups used to measure neurite outgrowth described in Figure 5A-D. [#p=0.034 % sprouting scrambled vs. DLK siRNA, one tailed unpaired Student’s t-test, n=4/group; *p=0.051 branching scrambled vs. DLK siRNA, two tailed unpaired Student’s t-test, n=4/group] (PNG 410 kb)
ESM 5
Supplemental Figure 5: (A-C) DUSP 1,4 siRNA knockdown, with or without capsaicin, scrambled siRNA with a DUSP inhibitor with or without capsaicin and DLK siRNA with a DUSP inhibitor with or without capsaicin were associated with reduced sprouting (A) and reduced branching (B). DUSP 1,4siRNA knockdown, with capsaicin, scrambled siRNA with a DUSP inhibitor with or without capsaicin and DLK siRNA with a DUSP inhibitor with or without capsaicin were associated with lower numbers of harvested neurons available for analysis (C). Results from A-C are from the same experimental groups used to measure neurite outgrowth described in Figure 4,5. [*p=0.015 ANOVA for % sprouting; *p=0.0002 ANOVA for branching; *p=0.045 for numbers; n=3 for carrier inhibitor, 3 for carrier capsaicin and 4 for all other groups] (PNG 158 kb)
Rights and permissions
About this article
Cite this article
Chandrasekhar, A., Komirishetty, P., Areti, A. et al. Dual Specificity Phosphatases Support Axon Plasticity and Viability. Mol Neurobiol 58, 391–407 (2021). https://doi.org/10.1007/s12035-020-02119-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02119-6