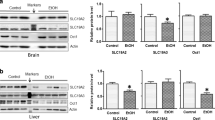
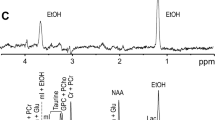
Abstract
Alcoholism is a chronic relapsing disorder defined by loss of control over excessive consumption of ethanol despite damaging effects on the liver and brain. We previously showed that the overexpression in mice of Dyrk1A (TgDyrk1A, for dual-specificity tyrosine (Y) phosphorylation-regulated kinase 1A) reduces the severity of alcohol mediated liver injury. Ethanol consumption has also been associated with increased brain glutamate concentration that led to therapies targeting glutamatergic receptors and normalization of glutamatergic neurotransmission. Interestingly, mice overexpressing Dyrk1A (TgDyrk1A mice) present a reduction of glutamatergic brain transmission, which we propose could be protective against alcohol intake. To answer this question, we investigated the ethanol preference in TgDyrk1A mice using a two-bottle choice paradigm. TgDyrk1A mice showed a non-significant decrease of voluntary ethanol intake and ethanol preference compared with wild-type mice. At the peripheral level, mice overexpressing Dyrk1A show lower ethanol plasma levels, indicating a faster ethanol metabolism. At the end of the protocol, lasting 21 days, brains were extracted for protein analysis. Ethanol reduced levels of the synaptic protein PSD-95 and increased the glutamate decarboxylase GAD65, specifically in the cortex of TgDyrk1A mice. Our results suggest that overexpression of DYRK1A may cause different ethanol-induced changes in the brain.







Similar content being viewed by others
Abbreviations
- BEC:
-
blood ethanol concentrations
- DYRK1A:
-
Dual-specificity tyrosine (Y) phosphorylation-regulated kinase 1A
- GABA:
-
Gamma amino butyric acid
- GAD:
-
Glutamic acid decarboxylase
- GLAST:
-
Glutamate aspartate transporter
- GLT1:
-
Glutamate transporter-1
- GluN2A:
-
Glutamate ionotropic receptor NMDA type subunit 2A
- NMDA:
-
N-methyl-D-aspartate
- NMDAR:
-
NMDA receptor
- PSD-95:
-
Post-synaptic density 95
- VGAT1:
-
Vesicular transporter of GABA
- VGLUT1:
-
Vesicular transporter of glutamate
- WT:
-
Wild-type
References
Spanagel R (2018) Aberrant choice behavior in alcoholism. Science 360:1298–1299
Harper C (2009) The neuropathology of alcohol-related brain damage. Alcohol Alcohol 44:136–140
Smith JE, Co C, McIntosh S, Cunningham CC (2008) Chronic binge-like moderate ethanol drinking in rats results in widespread decreases in brain serotonin, dopamine, and norepinephrine turnover rates reversed by ethanol intake. J Neurochem 105:2134–2155
Spanagel R (2009) Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev 89:649–705
Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M et al (2005) The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 11:35–42
Spanagel R, Kiefer F (2008) Drugs for relapse prevention of alcoholism: ten years of progress. Trends Pharmacol Sci 29:109–115
Holmes A, Spanagel R, Krystal JH (2013) Glutamatergic targets for new alcohol medications. Psychopharmacology 229:539–554
Abrahao KP, Salinas AG, Lovinger DM (2017) Alcohol and the brain: neuronal molecular targets, synapses, and circuits. Neuron 96:1223–1238
McCool BA (2011) Ethanol modulation of synaptic plasticity. Neuropharmacology 61:1097–1108
Altafaj X, Ortiz-Abalia J, Fernandez M, Potier MC, Laffaire J, Andreu N, Dierssen M, Gonzalez-Garcia C et al (2008) Increased NR2A expression and prolonged decay of NMDA-induced calcium transient in cerebellum of TgDyrk1A mice, a mouse model of down syndrome. Neurobiol Dis 32:377–384
Souchet B, Guedj F, Sahun I, Duchon A, Daubigney F, Badel A, Yanagawa Y, Barallobre MJ et al (2014) Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol Dis 69:65–75
Souchet B, Guedj F, Penke-Verdier Z, Daubigney F, Duchon A, Herault Y, Bizot JC, Janel N et al (2015) Pharmacological correction of excitation/inhibition imbalance in Down syndrome mouse models. Front Behav Neurosci 9:267
Lee SE, Lee Y, Lee GH (2019) The regulation of glutamic acid decarboxylases in GABA neurotransmission in the brain. Arch Pharm Res 42:1031–1039
Dang T, Duan WY, Yu B, Tong DL, Cheng C, Zhang YF, Wu W, Ye K et al (2018) Autism-associated Dyrk1a truncation mutants impair neuronal dendritic and spine growth and interfere with postnatal cortical development. Mol Psychiatry 23:747–758
Benavides-Piccione R, Dierssen M, Ballesteros-Yanez I, Martinez de Lagran M, Arbones M, Fotaki V, De Felipe J, Elston G (2005) Alterations in the phenotype of neocortical pyramidal cells in the Dyrk1A+/− mouse. Neurobiol Dis 20:115–122
Arranz J, Balducci E, Arató K, Sánchez-Elexpuru G, Najas S, Parras A, Rebollo E, Pijuan I et al (2019) Impaired development of neocortical circuits contributes to the neurological alterations in DYRK1A haploinsufficiency syndrome. Neurobiol Dis 127:210–222
Fotaki V, Dierssen M, Alcantara S, Martinez S, Marti E, Casas C, Visa J, Soriano E et al (2002) Dyrk1A haploinsufficiency affects viability and causes developmental delay and abnormal brain morphology in mice. Mol Cell Biol 22:6636–6647
Renon M, Legrand B, Blanc E, Daubigney F, Bokobza C, Mortreux M, Paul JL, Delabar JM et al (2016) Impact of Dyrk1A level on alcohol metabolism. Biochim Biophys Acta 1862:1495–1503
Guedj F, Pereira PL, Najas S, Barallobre MJ, Chabert C, Souchet B, Sebrie C, Verney C et al (2012) DYRK1A: A master regulatory protein controlling brain growth. Neurobiol Dis 46:190–203
Becker JB, Hu M (2008) Sex differences in drug abuse. Front Neuroendocrinol 29:36–47
Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, Schafer GL (1996) Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet 14:98–101
Kelai S, Renoir T, Chouchana L, Saurini F, Hanoun N, Hamon M, Lanfumey L (2008) Chronic voluntary ethanol intake hypersensitizes 5-HT(1A) autoreceptors in C57BL/6J mice. J Neurochem 107:1660–1670
Cui C, Noronha A, Morikawa H, Alvarez VA, Stuber GD, Szumlinski KK, Kash TL, Roberto M et al (2013) New insights on neurobiological mechanisms underlying alcohol addiction. Neuropharmacology 67:223–232
Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ (2012) Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One 7:e37541
Dierssen M, de Lagrán MM (2006) DYRK1A (dual-specificity tyrosine-phosphorylated and -regulated kinase 1A): a gene with dosage effect during development and neurogenesis. ScientificWorldJournal. 6:1911–1922
Martinez de Lagran M, Benavides-Piccione R, Ballesteros-Yanez I, Calvo M, Morales M, Fillat C, Defelipe J, Ramakers GJ et al (2012) Dyrk1A influences neuronal morphogenesis through regulation of cytoskeletal dynamics in mammalian cortical neurons. Cereb Cortex 22:2867–2877
Ruiz-Mejias M, Martinez de Lagran M, Mattia M, Castano-Prat P, Perez-Mendez L, Ciria-Suarez L, Gener T, Sancristobal B et al (2016) Overexpression of Dyrk1A, a down syndrome candidate. Decreases Excitability and Impairs Gamma Oscillations in the Prefrontal Cortex J Neurosci 36:3648–3659
Grau C, Arató K, Fernández-Fernández JM, Valderrama A, Sindreu C, Fillat C, Ferrer I, de la Luna S et al (2014) DYRK1A-mediated phosphorylation of GluN2A at Ser(1048) regulates the surface expression and channel activity of GluN1/GluN2A receptors. Front Cell Neurosci 8:331
Varodayan FP, Sidhu H, Kreifeldt M, Roberto M, Contet C (2018) Morphological and functional evidence of increased excitatory signaling in the prelimbic cortex during ethanol withdrawal. Neuropharmacology 133:470–480
Howell MD, Gottschall PE (2012) Altered synaptic marker abundance in the hippocampal stratum oriens of Ts65Dn mice is associated with exuberant expression of versican. ASN Neuro 4:e00073
Zhang J, Saur T, Duke AN, Grant SG, Platt DM, Rowlett JK, Isacson O, Yao WD (2014) Motor impairments, striatal degeneration, and altered dopamine-glutamate interplay in mice lacking PSD-95. J Neurogenet 28:98–111
Camp MC, Feyder M, Ihne J, Palachick B, Hurd B, Karlsson RM, Noronha B, Chen YC et al (2011) A novel role for PSD-95 in mediating ethanol intoxication, drinking and place preference. Addict Biol 16:428–439
Kleschevnikov AM, Belichenko PV, Gall J, George L, Nosheny R, Maloney MT, Salehi A, Mobley WC (2012) Increased efficiency of the GABAA and GABAB receptor-mediated neurotransmission in the Ts65Dn mouse model of Down syndrome. Neurobiol Dis 45:683–691
Blednov YA, Walker DL, Lyer SV, Homanics G, Harris AR (2010) Mice lacking Gad2 show altered behavioral effects of ethanol, flurazepam and gabaxadol. Addict Biol 15:45–61
Melendez RI, Hicks MP, Cagle SS, Kalivas PW (2005) Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res 29:326–333
Kryger R, Wilce PA (2010) The effects of alcoholism on the human basolateral amygdala. Neuroscience 167:361–371
Bellocchio EE, Reimer RJ, Fremeau RT Jr, Edwards RH (2000) Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science 289:957–960
Takamori S, Rhee JS, Rosenmund C, Jahn R (2000) Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature 407:189–194
Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD (2012) Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci 32:1884–1897
Kashem MA, Sultana N, Pow DV, Balcar VJ (2019) GLAST (GLutamate and ASpartate Transporter) in human prefrontal cortex; interactome in healthy brains and the expression of GLAST in brains of chronic alcoholics. Neurochem Int 125:111–116
Rao PS, Sari Y (2014) Effects of ceftriaxone on chronic ethanol consumption: a potential role for xCT and GLT1 modulation of glutamate levels in male P rats. J Mol Neurosci 54:71–77
Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SH, Wei Y, Sari Y (2014) Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav Neurosci 8:366
Sari Y, Sreemantula SN (2012) Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience 227:327–335
Ezquer F, Quintanilla ME, Morales P, Santapau D, Ezquer M, Kogan MJ, Salas-Huenuleo E, Herrera-Marschitz M et al (2019) Intranasal delivery of mesenchymal stem cell-derived exosomes reduces oxidative stress and markedly inhibits ethanol consumption and post-deprivation relapse drinking. Addict Biol 24:994–1007
Ahn KJ, Jeong HK, Choi HS, Ryoo SR, Kim YJ, Goo JS, Choi SY, Han JS et al (2006) DYRK1A BAC transgenic mice show altered synaptic plasticity with learning and memory defects. Neurobiol Dis 22:463–472
Acknowledgments
We thank Dr. Laurence Lanfumey for the helpful discussion. We thank the platform accommodation and animal testing of the animal house at the Institute Jacques Monod (University Paris Diderot) and the FlexStation3 Facility.
Funding
This work was supported by the IREB association. The group of MD is supported by the CRG Severo Ochoa excellence grant (SEV-2012-0208), the CIBER of Rare Diseases, and the “Secretaria d’Universitats i Recerca del Departament d’Economia I Coneixement de la Generalitat de Catalunya” (Grups consolidats 2017SGR595). We also acknowledge the support of the Spanish Ministry of Science, Innovation and Universities (MSIU) to the EMBL partnership, the Centro de Excelencia Severo Ochoa, and the CERCA Programme/Generalitat de Catalunya.
Author information
Authors and Affiliations
Contributions
MD and NJ designed study; MF, YCG, NK, and MML performed research; MF, YCG, MD, and NJ analyzed data; MD and NJ wrote the paper.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Fructuoso, M., Gu, Y.C., Kassis, N. et al. Ethanol-Induced Changes in Brain of Transgenic Mice Overexpressing DYRK1A. Mol Neurobiol 57, 3195–3205 (2020). https://doi.org/10.1007/s12035-020-01967-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01967-6