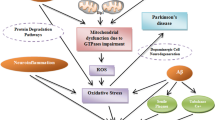
Abstract
Although the pathogenesis of neurodegenerative diseases is still widely unclear, various mechanisms have been proposed and several pieces of evidence are supportive for an important role of mitochondrial dysfunction. The present review provides a comprehensive and up-to-date overview about the role of mitochondria in the two most common neurodegenerative disorders: Alzheimer’s disease (AD) and Parkinson’s disease (PD). Mitochondrial involvement in AD is supported by clinical features like reduced glucose and oxygen brain metabolism and by numerous microscopic and molecular findings, including altered mitochondrial morphology, impaired respiratory chain function, and altered mitochondrial DNA. Furthermore, amyloid pathology and mitochondrial dysfunction seem to be bi-directionally correlated. Mitochondria have an even more remarkable role in PD. Several hints show that respiratory chain activity, in particular complex I, is impaired in the disease. Mitochondrial DNA alterations, involving deletions, point mutations, depletion, and altered maintenance, have been described. Mutations in genes directly implicated in mitochondrial functioning (like Parkin and PINK1) are responsible for rare genetic forms of the disease. A close connection between alpha-synuclein accumulation and mitochondrial dysfunction has been observed. Finally, mitochondria are involved also in atypical parkinsonisms, in particular multiple system atrophy. The available knowledge is still not sufficient to clearly state whether mitochondrial dysfunction plays a primary role in the very initial stages of these diseases or is secondary to other phenomena. However, the presented data strongly support the hypothesis that whatever the initial cause of neurodegeneration is, mitochondrial impairment has a critical role in maintaining and fostering the neurodegenerative process.



Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- APP:
-
amyloid precursor protein
- PET:
-
positron emission tomography
- mtDNA:
-
mitochondrial DNA
- CR:
-
control region
- ABAD:
-
Aβ-binding alcohol dehydrogenase
- UPRmt :
-
mitochondrial unfolded protein response
- SN:
-
substantia nigra
- α-syn:
-
alpha-synuclein
- MPTP:
-
1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine
- PEO:
-
progressive external ophthalmoplegia
- TOM20:
-
translocase of the outer membrane 20
- MAM:
-
mitochondria-associated endoplasmic reticulum membranes
- MSA:
-
multiple system atrophy
- CoQ10:
-
coenzyme Q10
References
Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10(4).
Friedman JR, Nunnari J (2014) Mitochondrial form and function. Nature. 505(7483):335–343
DiMauro S, Schon EA (2003) Mitochondrial respiratory-chain diseases. N Engl J Med 348(26):2656–2668
Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, Suomalainen A, Thorburn DR et al (2016) Mitochondrial diseases. Nat Rev Dis Primers 2:16080
Wallace DC (1999) Mitochondrial diseases in man and mouse. Science. 283(5407):1482–1488
Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev Dis Primers 1:15056
Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM (2016) Alzheimer’s disease. Lancet. 388(10043):505–517
Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81(2):741–766
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 297(5580):353–356
Swerdlow RH (2018) Mitochondria and mitochondrial cascades in Alzheimer’s disease. J Alzheimers Dis 62(3):1403–1416
Swerdlow RH, Burns JM, Khan SM (2010) The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis 20(Suppl 2):S265–S279
Ferris SH, de Leon MJ, Wolf AP, Farkas T, Christman DR, Reisberg B, Fowler JS, Macgregor R et al (1980) Positron emission tomography in the study of aging and senile dementia. Neurobiol Aging 1(2):127–131
Foster NL, Chase TN, Fedio P, Patronas NJ, Brooks RA, Di Chiro G (1983) Alzheimer’s disease: focal cortical changes shown by positron emission tomography. Neurology. 33(8):961–965
Friedland RP, Budinger TF, Ganz E, Yano Y, Mathis CA, Koss B, Ober BA, Huesman RH et al (1983) Regional cerebral metabolic alterations in dementia of the Alzheimer type: positron emission tomography with [18F]fluorodeoxyglucose. J Comput Assist Tomogr 7(4):590–598
de Leon MJ, Ferris SH, George AE, Christman DR, Fowler JS, Gentes C, Reisberg B, Gee B et al (1983) Positron emission tomographic studies of aging and Alzheimer disease. AJNR Am J Neuroradiol 4(3):568–571
Shokouhi S, Claassen D, Riddle W (2014) Imaging Brain Metabolism and Pathology in Alzheimer's Disease with Positron Emission Tomography. J Alzheimers Dis Parkinsonism 4(2):143
Frackowiak RS, Pozzilli C, Legg NJ, Du Boulay GH, Marshall J, Lenzi GL, Jones T (1981) Regional cerebral oxygen supply and utilization in dementia. A clinical and physiological study with oxygen-15 and positron tomography. Brain. 104(Pt 4):753–778
Fukuyama H, Ogawa M, Yamauchi H, Yamaguchi S, Kimura J, Yonekura Y, Konishi J (1994) Altered cerebral energy metabolism in Alzheimer’s disease: a PET study. J Nucl Med 35(1):1–6
Ishii K, Kitagaki H, Kono M, Mori E (1996) Decreased medial temporal oxygen metabolism in Alzheimer’s disease shown by PET. J Nucl Med 37(7):1159–1165
Tohgi H, Yonezawa H, Takahashi S, Sato N, Kato E, Kudo M, Hatano K, Sasaki T (1998) Cerebral blood flow and oxygen metabolism in senile dementia of Alzheimer’s type and vascular dementia with deep white matter changes. Neuroradiology. 40(3):131–137
Baloyannis SJ (2006) Mitochondrial alterations in Alzheimer’s disease. J Alzheimers Dis 9(2):119–126
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y et al (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21(9):3017–3023
Zhang L, Trushin S, Christensen TA, Bachmeier BV, Gateno B, Schroeder A, Yao J, Itoh K et al (2016) Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s disease. Sci Rep 6:18725
Gibson GE, Sheu KF, Blass JP, Baker A, Carlson KC, Harding B, Perrino P (1988) Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch Neurol 45(8):836–840
Perry EK, Perry RH, Tomlinson BE, Blessed G, Gibson PH (1980) Coenzyme A-acetylating enzymes in Alzheimer’s disease: possible cholinergic ‘compartment’ of pyruvate dehydrogenase. Neurosci Lett 18(1):105–110
Sorbi S, Bird ED, Blass JP (1983) Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol 13(1):72–78
Parker WD Jr, Filley CM, Parks JK (1990) Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 40(8):1302–1303
Cardoso SM, Proença MT, Santos S, Santana I, Oliveira CR (2004) Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurobiol Aging 25(1):105–110
Parker WD Jr, Parks J, Filley CM, Kleinschmidt-DeMasters BK (1994) Electron transport chain defects in Alzheimer’s disease brain. Neurology. 44(6):1090–1096
Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, DiStefano LM et al (1992) Brain cytochrome oxidase in Alzheimer’s disease. J Neurochem 59(2):776–779
Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM et al (2019) Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 22(3):401–412
Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF (2017) Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci 40(3):151–166
Martín-Maestro P, Gargini RA, Sproul A, García E, Antón LC, Noggle S, Arancio O, Avila J et al (2017) Mitophagy failure in fibroblasts and iPSC-derived neurons of Alzheimer’s disease-associated presenilin 1 Mutation. Front Mol Neurosci 10:291
Ye X, Sun X, Starovoytov V, Cai Q (2015) Parkin-mediated mitophagy in mutant hAPP neurons and Alzheimer’s disease patient brains. Hum Mol Genet 24(10):2938–2951
Keogh MJ, Chinnery PF (2015) Mitochondrial DNA mutations in neurodegeneration. Biochim Biophys Acta 1847(11):1401–1411
Coskun PE, Beal MF, Wallace DC (2004) Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A 101(29):10726–10731
Coskun PE, Wyrembak J, Derbereva O, Melkonian G, Doran E, Lott IT, Head E, Cotman CW et al (2010) Systemic mitochondrial dysfunction and the etiology of Alzheimer’s disease and down syndrome dementia. J Alzheimers Dis 20(Suppl 2):S293–S310
Podlesniy P, Figueiro-Silva J, Llado A, Antonell A, Sanchez-Valle R, Alcolea D, Lleo A, Molinuevo JL et al (2013) Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann Neurol 74(5):655–668
Rice AC, Keeney PM, Algarzae NK, Ladd AC, Thomas RR, Bennett JP Jr (2014) Mitochondrial DNA copy numbers in pyramidal neurons are decreased and mitochondrial biogenesis transcriptome signaling is disrupted in Alzheimer’s disease hippocampi. J Alzheimers Dis 40(2):319–330
Wei W, Keogh MJ, Wilson I, Coxhead J, Ryan S, Rollinson S, Griffin H, Kurzawa-Akinibi M et al (2017) Mitochondrial DNA point mutations and relative copy number in 1363 disease and control human brains. Acta Neuropathol Commun 5:13
van der Walt JM, Dementieva YA, Martin ER, Scott WK, Nicodemus KK, Kroner CC, Welsh-Bohmer KA, Saunders AM et al (2004) Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neurosci Lett 365(1):28–32
Santoro A, Balbi V, Balducci E, Pirazzini C, Rosini F, Tavano F, Achilli A, Siviero P et al (2010) Evidence for sub-haplogroup h5 of mitochondrial DNA as a risk factor for late onset Alzheimer’s disease. PLoS One 5(8):e12037
Lakatos A, Derbeneva O, Younes D, Keator D, Bakken T, Lvova M, Brandon M, Guffanti G et al (2010) Alzheimer’s disease neuroimaging initiative. Association between mitochondrial DNA variations and Alzheimer’s disease in the ADNI cohort. Neurobiol Aging 31(8):1355–1363
Maruszak A, Canter JA, Styczyńska M, Zekanowski C, Barcikowska M (2009) Mitochondrial haplogroup H and Alzheimer’s disease--is there a connection? Neurobiol Aging 30(11):1749–1755
Elson JL, Herrnstadt C, Preston G, Thal L, Morris CM, Edwardson JA, Beal MF, Turnbull DM et al (2006) Does the mitochondrial genome play a role in the etiology of Alzheimer’s disease? Hum Genet 119(3):241–254
Krüger J, Hinttala R, Majamaa K, Remes AM (2010) Mitochondrial DNA haplogroups in early-onset Alzheimer's disease and frontotemporal lobar degeneration. Mol Neurodegener 5:8
Mancuso M, Nardini M, Micheli D, Rocchi A, Nesti C, Giglioli NJ, Petrozzi L, Rossi C et al (2007) Lack of association between mtDNA haplogroups and Alzheimer’s disease in Tuscany. Neurol Sci 28(3):142–147
Chagnon P, Gee M, Filion M, Robitaille Y, Belouchi M, Gauvreau D (1999) Phylogenetic analysis of the mitochondrial genome indicates significant differences between patients with Alzheimer disease and controls in a French-Canadian founder population. Am J Med Genet 85(1):20–30
Ridge PG, Maxwell TJ, Corcoran CD, Norton MC, Tschanz JT, O'Brien E, Kerber RA, Cawthon RM et al (2012) Mitochondrial genomic analysis of late onset Alzheimer’s disease reveals protective haplogroups H6A1A/H6A1B: the Cache County study on memory in aging. PLoS One 7(9):e45134
Shoffner JM, Brown MD, Torroni A, Lott MT, Cabell MF, Mirra SS, Beal MF, Yang CC et al (1993) Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics. 17(1):171–184
Egensperger R, Kösel S, Schnopp NM, Mehraein P, Graeber MB (1997) Association of the mitochondrial tRNA(A4336G) mutation with Alzheimer’s and Parkinson’s diseases. Neuropathol Appl Neurobiol 23(4):315–321
Hutchin T, Cortopassi G (1995) A mitochondrial DNA clone is associated with increased risk for Alzheimer disease. Proc Natl Acad Sci U S A 92(15):6892–6895
García-Lozano JR, Mir P, Alberca R, Aguilera I, Gil Néciga E, Fernández-López O, Cayuela A, Núñez-Roldan A (2002) Mitochondrial DNA A4336G mutation in Alzheimer’s and Parkinson’s diseases. Eur Neurol 48(1):34–36
Wragg MA, Talbot CJ, Morris JC, Lendon CL, Goate AM (1995) No association found between Alzheimer’s disease and a mitochondrial tRNA glutamine gene variant. Neurosci Lett 201(2):107–110
Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, McKee AC, Beal MF, Graham BH, Wallace DC (1994) Marked changes in mitochondrial DNA deletion levels in Alzheimer brains. Genomics 23(2):471–476
Hamblet NS, Castora FJ (1997) Elevated levels of the Kearns-Sayre syndrome mitochondrial DNA deletion in temporal cortex of Alzheimer’s patients. Mutat Res 379(2):253–262
Chang SW, Zhang D, Chung HD, Zassenhaus HP (2000) The frequency of point mutations in mitochondrial DNA is elevated in the Alzheimer’s brain. Biochem Biophys Res Commun 273(1):203–208
Mawrin C, Kirches E, Krause G, Schneider-Stock R, Bogerts B, Vorwerk CK, Dietzmann K (2004) Region-specific analysis of mitochondrial DNA deletions in neurodegenerative disorders in humans. Neurosci Lett 357(2):111–114
Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF (2002) High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum Mol Genet 11(2):133–145
Hudson G, Sims R, Harold D, Chapman J, Hollingworth P, Gerrish A, Russo G, Hamshere M et al (2012) GERAD1 consortium. No consistent evidence for association between mtDNA variants and Alzheimer disease. Neurology. 78(14):1038–1042
Edland SD, Silverman JM, Peskind ER, Tsuang D, Wijsman E, Morris JC (1996) Increased risk of dementia in mothers of Alzheimer’s disease cases: evidence for maternal inheritance. Neurology. 47(1):254–256
Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, Tsui W, De Santi S et al (2007) Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A 104(48):19067–19072
Honea RA, Swerdlow RH, Vidoni ED, Goodwin J, Burns JM (2010) Reduced gray matter volume in normal adults with a maternal family history of Alzheimer disease. Neurology. 74(2):113–120
Mosconi L, Rinne JO, Tsui WH, Berti V, Li Y, Wang H, Murray J, Scheinin N et al (2010) Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer’s. Proc Natl Acad Sci U S A 107(13):5949–5954
Szabados T, Dul C, Majtényi K, Hargitai J, Pénzes Z, Urbanics R (2004) A chronic Alzheimer’s model evoked by mitochondrial poison sodium azide for pharmacological investigations. Behav Brain Res 154(1):31–40
Höglinger GU, Lannuzel A, Khondiker ME, Michel PP, Duyckaerts C, Féger J, Champy P, Prigent A et al (2005) The mitochondrial complex I inhibitor rotenone triggers a cerebral tauopathy. J Neurochem 95(4):930–939
Escobar-Khondiker M, Höllerhage M, Muriel MP, Champy P, Bach A, Depienne C, Respondek G, Yamada ES et al (2007) Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J Neurosci 27(29):7827–7837
Gabuzda D, Busciglio J, Chen LB, Matsudaira P, Yankner BA (1994) Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J Biol Chem 269(18):13623–13628
Gasparini L, Racchi M, Benussi L, Curti D, Binetti G, Bianchetti A, Trabucchi M, Govoni S (1997) Effect of energy shortage and oxidative stress on amyloid precursor protein metabolism in COS cells. Neurosci Lett 231(2):113–117
Kukreja L, Kujoth GC, Prolla TA, Van Leuven F, Vassar R (2014) Increased mtDNA mutations with aging promotes amyloid accumulation and brain atrophy in the APP/Ld transgenic mouse model of Alzheimer’s disease. Mol Neurodegener 9:16
Sheehan JP, Swerdlow RH, Miller SW, Davis RE, Parks JK, Parker WD, Tuttle JB (1997) Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer’s disease. J Neurosci 17(12):4612–4622
Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP Jr, Davis RE, Parker WD Jr (1997) Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology. 49(4):918–925
Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, Krebs CT, Bennett JC et al (2000) Alzheimer’s disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol 48(2):148–155
Silva DF, Selfridge JE, Lu J, E L, Roy N, Hutfles L, Burns JM, Michaelis EK, Yan S, Cardoso SM, Swerdlow RH. Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cybrid cell lines. Hum Mol Genet 2013;22(19):3931–3946.
Schon EA, Shoubridge EA, Moraes CT (1998) Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology. 51(1):326–327
Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O et al (2002) Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis 10(3):279–288
Pereira C, Santos MS, Oliveira C (1998) Mitochondrial function impairment induced by amyloid beta-peptide on PC12 cells. Neuroreport. 9(8):1749–1755
Canevari L, Clark JB, Bates TE (1999) Beta-amyloid fragment 25-35 selectively decreases complex IV activity in isolated mitochondria. FEBS Lett 457(1):131–134
Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA et al (2005) Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J Neurosci 25(3):672–679
Cardoso SM, Santos S, Swerdlow RH, Oliveira CR (2001) Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB J 15(8):1439–1441
Fukui H, Diaz F, Garcia S, Moraes CT (2007) Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 104(35):14163–14168
Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci 29(28):9090–9103
Wang X, Su B, Fujioka H, Zhu X (2008) Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol 173(2):470–482
Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X (2008) Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A 105(49):19318–19323
Manczak M, Calkins MJ, Reddy PH (2011) Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum Mol Genet 20(13):2495–2509
Beck JS, Mufson EJ, Counts SE (2016) Evidence for mitochondrial UPR gene activation in familial and sporadic Alzheimer’s disease. Curr Alzheimer Res 13(6):610–614
Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D'Amico D, Moullan N, Potenza F et al (2017) Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature. 552(7684):187–193
Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D et al (2005) Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J 19(14):2040–2041
Chen JX, Yan SS (2010) Role of mitochondrial amyloid-beta in Alzheimer’s disease. J Alzheimers Dis 20(Suppl 2):S569–S578
Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK (2006) Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci 26(35):9057–9068
Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG (2003) Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol 161(1):41–54
Pagani L, Eckert A (2011) Amyloid-beta interaction with mitochondria. Int J Alzheimers Dis 2011:925050
Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X et al (2004) ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 304(5669):448–452
Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C et al (2008) Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med 14(10):1097–1105
Supnet C, Bezprozvanny I (2010) Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer’s disease. J Alzheimers Dis 20(Suppl 2):S487–S498
Ballatore C, Lee VM, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8(9):663–672
David DC, Hauptmann S, Scherping I, Schuessel K, Keil U, Rizzu P, Ravid R, Dröse S et al (2005) Proteomic and functional analyses reveal a mitochondrial dysfunction inP301L tau transgenic mice. J Biol Chem 280(25):23802–23814
Eckert A, Nisbet R, Grimm A, Götz J (2014) March separate, strike together—role of phosphorylated TAU in mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta 1842(8):1258–1266
Cheng Y, Bai F (2018) The Association of tau with mitochondrial dysunction in Alzheimer’s disease. Front Neurosci 12:163
Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E (1998) Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. J Cell Biol 143(3):777–794
Kopeikina KJ, Carlson GA, Pitstick R, Ludvigson AE, Peters A, Luebke JI, Koffie RM, Frosch MP et al (2011) Tau accumulation causes mitochondrial distribution deficits in neurons in a mouse model of tauopathy and in human Alzheimer’s disease brain. Am J Pathol 179(4):2071–2082
Manczak M, Reddy PH (2012) Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet 21(11):2538–2547
Li XC, Hu Y, Wang ZH, Luo Y, Zhang Y, Liu X, Feng Q, Wang Q et al (2016) Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Sci Rep 6:24756
Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H et al (2009) Amylois-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci U S A 106(47):20057–20062
Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD (2009) Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 106(34):14670–14675
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang E (2017) Parkinson disease. Nat Rev Dis Primers 3:17013
Schapira AH (2008) Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol 7(1):97–109
Giannoccaro MP, La Morgia C, Rizzo G, Carelli V (2017) Mitochondrial DNA and primary mitochondrial dysfunction in Parkinson's disease. Mov Disord 32(3):346–363
Rango M, Bresolin N. Brain mitochondria, Aging, and Parkinson’s Disease. Genes (Basel). 2018;9(5).
Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1(8649):1269
Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD (1990) Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson’s disease. J Neurochem 55(6):2142–2145
Mann VM, Cooper JM, Krige D, Daniel SE, Schapira AH, Marsden CD (1992) Brain, skeletal muscle and platelet homogenate mitochondrial function in Parkinson’s disease. Brain. 115(Pt 2):333–342
Janetzky B, Hauck S, Youdim MB, Riederer P, Jellinger K, Pantucek F, Zöchling R, Boissl KW et al (1994) Unaltered aconitase activity, but decreased complex I activity in substantia nigra pars compacta of patients with Parkinson’s disease. Neurosci Lett 169(1–2):126–128
Mann VM, Cooper JM, Daniel SE, Srai K, Jenner P, Marsden CD, Schapira AH (1994) Complex I, iron, and ferritin in Parkinson’s disease substantia nigra. Ann Neurol 36(6):876–881
Cardellach F, Martí MJ, Fernández-Solá J, Marín C, Hoek JB, Tolosa E, Urbano-Márquez A (1993) Mitochondrial respiratory chain activity in skeletal muscle from patients with Parkinson’s disease. Neurology. 43(11):2258–2262
Shoffner JM, Watts RL, Juncos JL, Torroni A, Wallace DC (1991) Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol 30(3):332–339
Winkler-Stuck K, Kirches E, Mawrin C, Dietzmann K, Lins H, Wallesch CW, Kunz WS, Wiedemann FR (2005) Re-evaluation of the dysfunction of mitochondrial respiratory chain in skeletal muscle of patients with Parkinson’s disease. J Neural Transm (Vienna) 112(4):499–518
DiDonato S, Zeviani M, Giovannini P, Savarese N, Rimoldi M, Mariotti C, Girotti F, Caraceni T (1993) Respiratory chain and mitochondrial DNA in muscle and brain in Parkinson’s disease patients. Neurology. 43(11):2262–2268
Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, Shults CW (1995) Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson’s disease. Ann Neurol 37(6):714–722
Krige D, Carroll MT, Cooper JM, Marsden CD, Schapira AH (1992) Platelet mitochondrial function in Parkinson’s disease. The Royal Kings and Queens Parkinson disease research group. Ann Neurol 32(6):782–788
Parker WD Jr, Boyson SJ, Parks JK (1989) Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol 26(6):719–723
Martín MA, Molina JA, Jiménez-Jiménez FJ, Benito-León J, Ortí-Pareja M, Campos Y, Arenas J (1996) Respiratory-chain enzyme activities in isolated mitochondria of lymphocytes from untreated Parkinson’s disease patients. Grupo-Centro de Trastornos del Movimento Neurology 46(5):1343–1346
Müftüoglu M, Elibol B, Dalmizrak O, Ercan A, Kulaksiz G, Ogüs H, Dalkara T, Ozer N (2004) Mitochondrial complex I and IV activities in leukocytes from patients with parkin mutations. Mov Disord 19(5):544–548
Yoshino H, Nakagawa-Hattori Y, Kondo T, Mizuno Y (1992) Mitochondrial complex I and II activities of lymphocytes and platelets in Parkinson’s disease. J Neural Transm Park Dis Dement Sect 4(1):27–34
Hattori N, Tanaka M, Ozawa T, Mizuno Y (1991) Immunohistochemical studies on complexes I, II, III, and IV of mitochondria in Parkinson’s disease. Ann Neurol 30(4):563–571
Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T et al (1989) Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem Biophys Res Commun 163(3):1450–1455
Gu M, Cooper JM, Taanman JW, Schapira AH (1998) Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Ann Neurol 44(2):177–186
Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP Jr, Davis RE et al (1996) Origin and functional consequences of the complex I defect in Parkinson's disease. Ann Neurol 40(4):663–671
Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, Kopin IJ (1979) Chronic parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res 1(3):249–254
Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic parkinsonism in humans due to a product of meperidine -analog synthesis. Science. 219(4587):979–980
Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ (1983) A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A 80(14):4546–4550
Heikkila RE, Hess A, Duvoisin RC (1984) Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science 224(4656):1451–1453
Beal MF (2001) Experimental models of Parkinson’s disease. Nat Rev Neurosci 2(5):325–334
Meredith GE, Rademacher DJ (2011) MPTP mouse models of Parkinson’s disease: an update. J Park Dis 1(1):19–33
Porras G, Li Q, Bezard E (2012) Modeling Parkinson’s disease in Primates: the MPTP model. Cold Spring Harb Perspect Med 2(3):a009308
Nicklas WJ, Vyas I, Heikkila RE (1985) Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci 36(26):2503–2508
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron. 39(6):889–909
Javitch JA, D'Amato RJ, Strittmatter SM, Snyder SH (1985) Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A 82(7):2173–2177
Przedborski S, Tieu K, Perier C, Vila M (2004) MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J Bioenerg Biomembr 36(4):375–379
Degli EM (1998) Inhibitors of NADH-ubiquinone reductase: an overview. Biochim Biophys Acta 1364(2):222–235
Jenner P (2001) Parkinson’s disease, pesticides and mitochondrial dysfunction. Trends Neurosci 24(5):245–247
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3(12):1301–1306
Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT (2002) An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci 22(16):7006–7015
Höglinger GU, Féger J, Prigent A, Michel PP, Parain K, Champy P, Ruberg M, Oertel WH et al (2003) Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J Neurochem 84(3):491–502
Choi WS, Kruse SE, Palmiter RD, Xia Z (2008) Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A 105(39):15136–15141
Champy P, Höglinger GU, Féger J, Gleye C, Hocquemiller R, Laurens A, Guérineau V, Laprévote O et al (2004) Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: possible relevance for atypical parkinsonism in Guadeloupe. J Neurochem 88(1):63–69
Ikebe S, Tanaka M, Ohno K, Sato W, Hattori K, Kondo T, Mizuno Y, Ozawa T (1990) Increase of deleted mitochondrial DNA in the striatum in Parkinson’s disease and senescence. Biochem Biophys Res Commun 170(3):1044–1048
Ozawa T, Tanaka M, Ikebe S, Ohno K, Kondo T, Mizuno Y (1990) Quantitative determination of deleted mitochondrial DNA relative to normal DNA in parkinsonian striatum by a kinetic PCR analysis. Biochem Biophys Res Commun 172(2):483–489
Lestienne P, Nelson J, Riederer P, Jellinger K, Reichmann H (1990) Normal mitochondrial genome in brain from patients with Parkinson’s disease and complex I defect. J Neurochem 55(5):1810–1812
Mann VM, Cooper JM, Schapira AH (1992) Quantitation of a mitochondrial DNA deletion in Parkinson's disease. FEBS Lett 299(3):218–222
Schapira AH, Holt IJ, Sweeney M, Harding AE, Jenner P, Marsden CD (1990) Mitochondrial DNA analysis in Parkinson's disease. Mov Disord 5(4):294–297
Gu G, Reyes PE, Golden GT, Woltjer RL, Hulette C, Montine TJ, Zhang J (2002) Mitochondrial DNA deletions/rearrangements in parkinson disease and related neurodegenerative disorders. J Neuropathol Exp Neurol 61(7):634–639
Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS et al (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38(5):515–517
Dölle C, Flønes I, Nido GS, Miletic H, Osuagwu N, Kristoffersen S, Lilleng PK, Larsen JP et al (2016) Defective mitochondrial DNA homeostasis in the substantia nigra in Parkinson disease. Nat Commun 7:13548
Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 38(5):518–520
Brown MD, Shoffner JM, Kim YL, Jun AS, Graham BH, Cabell MF, Gurley DS, Wallace DC (1996) Mitochondrial DNA sequence analysis of four Alzheimer’s and Parkinson’s disease patients. Am J Med Genet 61(3):283–289
Ikebe S, Tanaka M, Ozawa T (1995) Point mutations of mitochondrial genome in Parkinson’s disease. Brain Res Mol Brain Res 28(2):281–295
Kapsa RM, Jean-Francois MJ, Lertrit P, Weng S, Siregar N, Ojaimi J, Donnan G, Masters C et al (1996) Mitochondrial DNA polymorphism in substantia nigra. J Neurol Sci 144(1–2):204–211
Kösel S, Grasbon-Frodl EM, Mautsch U, Egensperger R, von Eitzen U, Frishman D, Hofmann S, Gerbitz KD et al (1998) Novel mutations of mitochondrial complex I in pathologically proven Parkinson disease. Neurogenetics. 1(3):197–204
Richter G, Sonnenschein A, Grünewald T, Reichmann H, Janetzky B (2002) Novel mitochondrial DNA mutations in Parkinson’s disease. J Neural Transm (Vienna) 109(5–6):721–729
Parker WD Jr, Parks JK (2005) Mitochondrial ND5 mutations in idiopathic Parkinson's disease. Biochem Biophys Res Commun 326(3):667–669
Kösel S, Grasbon-Frodl EM, Hagenah JM, Graeber MB, Vieregge P (2000) Parkinson disease: analysis of mitochondrial DNA in monozygotic twins. Neurogenetics. 2(4):227–230
Vives-Bauza C, Andreu AL, Manfredi G, Beal MF, Janetzky B, Gruenewald TH, Lin MT (2002) Sequence analysis of the entire mitochondrial genome in Parkinson’s disease. Biochem Biophys Res Commun 290(5):1593–1601
Simon DK, Mayeux R, Marder K, Kowall NW, Beal MF, Johns DR (2000) Mitochondrial DNA mutations in complex I and tRNA genes in Parkinson’s disease. Neurology. 54(3):703–709
Coxhead J, Kurzawa-Akanbi M, Hussain R, Pyle A, Chinnery P, Hudson G. Somatic mtDNA variation is an important component of Parkinson’s disease. Neurobiol Aging. 2016;38:217.e1–217.e6.
Lin MT, Cantuti-Castelvetri I, Zheng K, Jackson KE, Tan YB, Arzberger T, Lees AJ, Betensky RA et al (2012) Somatic mitochondrial DNA mutations in early Parkinson and incidental Lewy body disease. Ann Neurol 71(6):850–854
van der Walt JM, Nicodemus KK, Martin ER, Scott WK, Nance MA, Watts RL, Hubble JP, Haines JL et al (2003) Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am J Hum Genet 72(4):804–811
Pyle A, Foltynie T, Tiangyou W, Lambert C, Keers SM, Allcock LM, Davison J, Lewis SJ et al (2005) Mitochondrial DNA haplogroup cluster UKJT reduces the risk of PD. Ann Neurol 57(4):564–567
Ghezzi D, Marelli C, Achilli A, Goldwurm S, Pezzoli G, Barone P, Pellecchia MT, Stanzione P et al (2005) Mitochondrial DNA haplogroup K is associated with a lower risk of Parkinson’s disease in Italians. Eur J Hum Genet 13(6):748–752
Khusnutdinova E, Gilyazova I, Ruiz-Pesini E, Derbeneva O, Khusainova R, Khidiyatova I, Magzhanov R, Wallace DC (2008) A mitochondrial etiology of neurodegenerative diseases: evidence from Parkinson’s disease. Ann N Y Acad Sci 1147:1–20
Ross OA, McCormack R, Maxwell LD, Duguid RA, Quinn DJ, Barnett YA, Rea IM, El-Agnaf OM et al (2003) mt4216C variant in linkage with the mtDNA TJ cluster may confer a susceptibility to mitochondrial dysfunction resulting in an increased risk of Parkinson’s disease in the Irish. Exp Gerontol 38(4):397–405
Hudson G, Nalls M, Evans JR, Breen DP, Winder-Rhodes S, Morrison KE, Morris HR, Williams-Gray CH et al (2013) Two-stage association study and meta-analysis of mitochondrial DNA variants in Parkinson disease. Neurology. 80(22):2042–2048
Pyle A, Brennan R, Kurzawa-Akanbi M, Yarnall A, Thouin A, Mollenhauer B, Burn D, Chinnery PF et al (2015) Reduced cerebrospinal fluid mitochondrial DNA is a biomarker for early-stage Parkinson's disease. Ann Neurol 78(6):1000–1004
Grünewald A, Rygiel KA, Hepplewhite PD, Morris CM, Picard M, Turnbull DM (2016) Mitochondrial DNA depletion in respiratory chain-deficient Parkinson disease neurons. Ann Neurol 79(3):366–378
Pyle A, Anugrha H, Kurzawa-Akanbi M, Yarnall A, Burn D, Hudson G (2016) Reduced mitochondrial DNA copy number is a biomarker of Parkinson’s disease. Neurobiol Aging 38:216.e7–216.e10
Van Goethem G, Dermaut B, Löfgren A, Martin JJ, Van Broeckhoven C (2001) Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet 28(3):211–212
Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I et al (2004) Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet. 364(9437):875–882
Mancuso M, Filosto M, Oh SJ, DiMauro S (2004) A novel polymerase gamma mutation in a family with ophthalmoplegia, neuropathy, and parkinsonism. Arch Neurol 61(11):1777–1779
Hudson G, Schaefer AM, Taylor RW, Tiangyou W, Gibson A, Venables G, Griffiths P, Burn DJ et al (2007) Mutation of the linker region of the polymerase gamma-1 (POLG1) gene associated with progressive external ophthalmoplegia and parkinsonism. Arch Neurol 64(4):553–557
Invernizzi F, Varanese S, Thomas A, Carrara F, Onofrj M, Zeviani M (2008) Two novel POLG1 mutations in a patient with progressive external ophthalmoplegia, levodopa-responsive pseudo-orthostatic tremor and parkinsonism. Neuromuscul Disord 18(6):460–464
Dolhun R, Presant EM, Hedera P (2013) Novel polymerase gamma (POLG1) gene mutation in the linker domain associated with parkinsonism. BMC Neurol 13:92
Miguel R, Gago MF, Martins J, Barros P, Vale J, Rosas MJ (2014) POLG1-related levodopa-responsive parkinsonism. Clin Neurol Neurosurg 126:47–54
Remes AM, Hinttala R, Kärppä M, Soini H, Takalo R, Uusimaa J, Majamaa K (2008) Parkinsonism associated with the homozygous W748S mutation in the POLG1 gene. Parkinsonism Relat Disord 14(8):652–654
Synofzik M, Asmus F, Reimold M, Schöls L, Berg D (2010) Sustained dopaminergic response of parkinsonism and depression in POLG-associated parkinsonism. Mov Disord 25(2):243–245
Davidzon G, Greene P, Mancuso M, Klos KJ, Ahlskog JE, Hirano M, DiMauro S (2006) Early-onset familial parkinsonism due to POLG mutations. Ann Neurol 59(5):859–862
Betts-Henderson J, Jaros E, Krishnan KJ, Perry RH, Reeve AK, Schaefer AM, Taylor RW, Turnbull DM (2009) Alpha-synuclein pathology and parkinsonism associated with POLG1 mutations and multiple mitochondrial DNA deletions. Neuropathol Appl Neurobiol 35(1):120–124
Melberg A, Nennesmo I, Moslemi AR, Kollberg G, Luoma P, Suomalainen A, Holme E, Oldfors A (2005) Alzheimer pathology associated with POLG1 mutation, multiple mtDNA deletions, and APOE4/4: premature ageing or just coincidence? Acta Neuropathol 110(3):315–316
Tzoulis C, Tran GT, Schwarzlmüller T, Specht K, Haugarvoll K, Balafkan N, Lilleng PK, Miletic H et al (2013) Severe nigrostriatal degeneration without clinical parkinsonism in patients with polymerase gamma mutations. Brain. 136(Pt 8):2393–2404
Gui YX, Xu ZP, Lv W, Liu HM, Zhao JJ, Hu XY (2012) Association of mitochondrial DNA polymerase γ gene POLG1 polymorphisms with parkinsonism in Chinese populations. PLoS One 7(12):e50086
Hudson G, Tiangyou W, Stutt A, Eccles M, Robinson L, Burn DJ, Chinnery PF (2009) No association between common POLG1 variants and sporadic idiopathic Parkinson’s disease. Mov Disord 24(7):1092–1094
Luoma PT, Eerola J, Ahola S, Hakonen AH, Hellström O, Kivistö KT, Tienari PJ, Suomalainen A (2007) Mitochondrial DNA polymerase gamma variants in idiopathic sporadic Parkinson disease. Neurology. 69(11):1152–1159
Tiangyou W, Hudson G, Ghezzi D, Ferrari G, Zeviani M, Burn DJ, Chinnery PF (2006) POLG1 in idiopathic Parkinson disease. Neurology. 67(9):1698–1700
Ylönen S, Ylikotila P, Siitonen A, Finnilä S, Autere J, Majamaa K (2013) Variations of mitochondrial DNA polymerase γ in patients with Parkinson’s disease. J Neurol 260(12):3144–3149
Anvret A, Westerlund M, Sydow O, Willows T, Lind C, Galter D, Belin AC (2010) Variations of the CAG trinucleotide repeat in DNA polymerase γ (POLG1) is associated with Parkinson’s disease in Sweden. Neurosci Lett 485(2):117–120
Balafkan N, Tzoulis C, Müller B, Haugarvoll K, Tysnes OB, Larsen JP, Bindoff LA (2012) Number of CAG repeats in POLG1 and its association with Parkinson disease in the Norwegian population. Mitochondrion. 12(6):640–643
Eerola J, Luoma PT, Peuralinna T, Scholz S, Paisan-Ruiz C, Suomalainen A, Singleton AB, Tienari PJ (2010) POLG1 polyglutamine tract variants associated with Parkinson’s disease. Neurosci Lett 477(1):1–5
Bentley SR, Shan J, Todorovic M, Wood SA, Mellick GD (2014) Rare POLG1 CAG variants do not influence Parkinson's disease or polymerase gamma function. Mitochondrion. 15:65–68
Taanman JW, Schapira AH (2005) Analysis of the trinucleotide CAG repeat from the DNA polymerase gamma gene (POLG) in patients with Parkinson’s disease. Neurosci Lett 376(1):56–59
Baloh RH, Salavaggione E, Milbrandt J, Pestronk A (2007) Familial parkinsonism and ophthalmoplegia from a mutation in the mitochondrial DNA helicase twinkle. Arch Neurol 64(7):998–1000
Kiferle L, Orsucci D, Mancuso M, Lo Gerfo A, Petrozzi L, Siciliano G, Ceravolo R, Bonuccelli U (2013) Twinkle mutation in an Italian family with external progressive ophthalmoplegia and parkinsonism: a case report and an update on the state of art. Neurosci Lett 556:1–4
Vandenberghe W, Van Laere K, Debruyne F, Van Broeckhoven C, Van Goethem G (2009) Neurodegenerative parkinsonism and progressive external ophthalmoplegia with a twinkle mutation. Mov Disord 24(2):308–309
Spelbrink JN, Li FY, Tiranti V, Nikali K, Yuan QP, Tariq M, Wanrooij S, Garrido N et al (2001) Human mitochondrial DNA deletions associated with mutations in the gene encoding twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat Genet 28(3):223–231
Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y et al (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 392(6676):605–608
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D et al (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 304(5674):1158–1160
Nguyen TN, Padman BS, Lazarou M (2016) Deciphering the molecular signals of PINK1/Parkin mitophagy. Trends Cell Biol 26(10):733–744
Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA et al (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 441(7097):1162–1166
Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J et al (2006) Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 441(7097):1157–1161
Charan RA, LaVoie MJ (2015) Pathologic and therapeutic implications for the cell biology of parkin. Mol Cell Neurosci 66(Pt A):62–71
Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B (2008) Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A 105(19):7070–7075
Deng H, Dodson MW, Huang H, Guo M (2008) The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A 105(38):14503–14508
Ordonez DG, Lee MK, Feany MB (2018) α-synuclein induces mitochondrial dysfunction through spectrin and the actin cytoskeleton. Neuron 97(1):108–124.e6
Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lämmermann K, Brunner B, Kurz-Drexler A et al (2009) Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem 284(34):22938–22951
Rappold PM, Cui M, Grima JC, Fan RZ, de Mesy-Bentley KL, Chen L, Zhuang X, Bowers WJ et al (2014) Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat Commun 5:5244
Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J (2004) Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem 279(18):18614–18622
Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ (2003) Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A 100(7):4078–4083
Cooper O, Seo H, Andrabi S, Guardia-Laguarta C, Graziotto J, Sundberg M, McLean JR, Carrillo-Reid L et al (2012) Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Sci Transl Med 4(141):141ra90
Imaizumi Y, Okada Y, Akamatsu W, Koike M, Kuzumaki N, Hayakawa H, Nihira T, Kobayashi T et al (2012) Mitochondrial dysfunction associated with increased oxidative stress and α-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol Brain 5:35
Jiang H, Ren Y, Yuen EY, Zhong P, Ghaedi M, Hu Z, Azabdaftari G, Nakaso K et al (2012) Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun 3:668
Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D (2011) Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci 31(16):5970–5976
Shaltouki A, Sivapatham R, Pei Y, Gerencser AA, Momčilović O, Rao MS, Zeng X (2015) Mitochondrial alterations by PARKIN in dopaminergic neurons using PARK2 patient-specific and PARK2 knockout isogenic iPSC lines. Stem Cell Reports 4(5):847–859
Suzuki S, Akamatsu W, Kisa F, Sone T, Ishikawa KI, Kuzumaki N, Katayama H, Miyawaki A et al (2017) Efficient induction of dopaminergic neuron differentiation from induced pluripotent stem cells reveals impaired mitophagy in PARK2 neurons. Biochem Biophys Res Commun 483(1):88–93
Tabata Y, Imaizumi Y, Sugawara M, Andoh-Noda T, Banno S, Chai M, Sone T, Yamazaki K et al (2018) T-type calcium channels determine the vulnerability of dopaminergic neurons to mitochondrial stress in familial Parkinson disease. Stem Cell Reports 11(5):1171–1184
Exner N, Treske B, Paquet D, Holmström K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D et al (2007) Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci 27(45):12413–12418
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature. 388(6645):839–840
Bendor JT, Logan TP, Edwards RH (2013) The function of α-synuclein. Neuron. 79(6):1044–1066
Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14(1):38–48
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841.
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 276(5321):2045–2047
Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT et al (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18(2):106–108
Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J et al (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55(2):164–173
Subramaniam SR, Vergnes L, Franich NR, Reue K, Chesselet MF (2014) Region specific mitochondrial impairment in mice with widespread overexpression of alpha-synuclein. Neurobiol Dis 70:204–213
Li L, Nadanaciva S, Berger Z, Shen W, Paumier K, Schwartz J, Mou K, Loos P et al (2013) Human A53T α-synuclein causes reversible deficits in mitochondrial function and dynamics in primary mouse cortical neurons. PLoS One 8(12):e85815
Luth ES, Stavrovskaya IG, Bartels T, Kristal BS, Selkoe DJ (2014) Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J Biol Chem 289(31):21490–21507
Banerjee K, Sinha M, Pham Cle L, Jana S, Chanda D, Cappai R, Chakrabarti S (2010) Alpha-synuclein induced membrane depolarization and loss of phosphorylation capacity of isolated rat brain mitochondria: implications in Parkinson’s disease. FEBS Lett 584(8):1571–1576
Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P (2008) Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci 65(7–8):1272–1284
Tapias V, Hu X, Luk KC, Sanders LH, Lee VM, Greenamyre JT (2017) Synthetic alpha-synuclein fibrils cause mitochondrial impairment and selective dopamine neurodegeneration in part via iNOS-mediated nitric oxide production. Cell Mol Life Sci 74(15):2851–2874
Chinta SJ, Mallajosyula JK, Rane A, Andersen JK (2010) Mitochondrial α-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett 486(3):235–239
Loeb V, Yakunin E, Saada A, Sharon R (2010) The transgenic overexpression of alpha-synuclein and not its related pathology associates with complex I inhibition. J Biol Chem 285(10):7334–7343
Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK (2008) Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283(14):9089–9100
Reeve AK, Ludtmann MH, Angelova PR, Simcox EM, Horrocks MH, Klenerman D, Gandhi S, Turnbull DM et al (2015) Aggregated α-synuclein and complex I deficiency: exploration of their relationship in differentiated neurons. Cell Death Dis 6:e1820
Liu G, Zhang C, Yin J, Li X, Cheng F, Li Y, Yang H, Uéda K et al (2009) alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci Lett 454(3):187–192
Song DD, Shults CW, Sisk A, Rockenstein E, Masliah E (2004) Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp Neurol 186(2):158–172
Song LK, Ma KL, Yuan YH, Mu Z, Song XY, Niu F, Han N, Chen NH (2015) Targeted overexpression of α-Synuclein by rAAV2/1 vectors induces progressive nigrostriatal degeneration and increases vulnerability to MPTP in mouse. PLoS One 10(6):e0131281
Nieto M, Gil-Bea FJ, Dalfó E, Cuadrado M, Cabodevilla F, Sánchez B, Catena S, Sesma T et al (2006) Increased sensitivity to MPTP in human alpha-synuclein A30P transgenic mice. Neurobiol Aging 27(6):848–856
Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K et al (2002) Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A 99(22):14524–14529
Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante RJ, Kowall NW et al (2006) Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis 21(3):541–548
Fountaine TM, Wade-Martins R (2007) RNA interference-mediated knockdown of alpha-synuclein protects human dopaminergic neuroblastoma cells from MPP(+) toxicity and reduces dopamine transport. J Neurosci Res 85(2):351–363
Cannon JR, Geghman KD, Tapias V, Sew T, Dail MK, Li C, Greenamyre JT (2013) Expression of human E46K-mutated α-synuclein in BAC-transgenic rats replicates early-stage Parkinson’s disease features and enhances vulnerability to mitochondrial impairment. Exp Neurol 240:44–56
Shavali S, Brown-Borg HM, Ebadi M, Porter J (2008) Mitochondrial localization of alpha-synuclein protein in alpha-synuclein overexpressing cells. Neurosci Lett 439(2):125–128
Zharikov AD, Cannon JR, Tapias V, Bai Q, Horowitz MP, Shah V, El Ayadi A, Hastings TG et al (2015) shRNA targeting α-synuclein prevents neurodegeneration in a Parkinson's disease model. J Clin Invest 125(7):2721–2735
Fornai F, Schlüter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL et al (2005) Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci U S A 102(9):3413–3418
Ludtmann MH, Angelova PR, Ninkina NN, Gandhi S, Buchman VL, Abramov AY (2016) Monomeric alpha-synuclein exerts a physiological role on brain ATP synthase. J Neurosci 36(41):10510–10521
Di Maio R, Barrett PJ, Hoffman EK, Barrett CW, Zharikov A, Borah A, Hu X, McCoy J et al (2016) α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci Transl Med 8(342):342ra78
Bender A, Desplats P, Spencer B, Rockenstein E, Adame A, Elstner M, Laub C, Mueller S et al (2013) TOM40 mediates mitochondrial dysfunction induced by α-synuclein accumulation in Parkinson’s disease. PLoS One 8(4):e62277
Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, Nuscher B, Bartels T et al (2010) Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J 29(20):3571–3589
Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J et al (2011) Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem 286(23):20710–20726
Guardia-Laguarta C, Area-Gomez E, Rüb C, Liu Y, Magrané J, Becker D, Voos W, Schon EA et al (2014) α-Synuclein is localized to mitochondria-associated ER membranes. J Neurosci 34(1):249–259
Guardia-Laguarta C, Area-Gomez E, Schon EA, Przedborski S (2015) A new role for α-synuclein in Parkinson’s disease: alteration of ER-mitochondrial communication. Mov Disord 30(8):1026–1033
Ubhi K, Low P, Masliah E (2011) Multiple system atrophy: a clinical and neuropathological perspective. Trends Neurosci 34(11):581–590
Monzio Compagnoni G, Di Fonzo A (2019) Understanding the pathogenesis of multiple system atrophy: state of the art and future perspectives. Acta Neuropathol Commun 7(1):113
Blin O, Desnuelle C, Rascol O, Borg M, Peyro Saint Paul H, Azulay JP, Billé F, Figarella D et al (1994) Mitochondrial respiratory failure in skeletal muscle from patients with Parkinson's disease and multiple system atrophy. J Neurol Sci 125(1):95–101
Gu M, Gash MT, Cooper JM, Wenning GK, Daniel SE, Quinn NP, Marsden CD, Schapira AH (1997) Mitochondrial respiratory chain function in multiple system atrophy. Mov Disord 12(3):418–422
Multiple-System Atrophy Research Collaboration (2013) Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med 369(3):233–244
Quinzii CM, Hirano M (2011) Primary and secondary CoQ(10) deficiencies in humans. Biofactors. 37(5):361–365
Quinzii C, Naini A, Salviati L, Trevisson E, Navas P, Dimauro S, Hirano M (2006) A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am J Hum Genet 78(2):345–349
Lin CH, Tan EK, Yang CC, Yi Z, Wu RM (2015) COQ2 gene variants associate with cerebellar subtype of multiple system atrophy in Chinese. Mov Disord 30(3):436–437
Ogaki K, Fujioka S, Heckman MG, Rayaprolu S, Soto-Ortolaza AI, Labbé C, Walton RL, Lorenzo-Betancor O et al (2014) Analysis of COQ2 gene in multiple system atrophy. Mol Neurodegener 9:44
Ronchi D, Di Biase E, Franco G, Melzi V, Del Sorbo F, Elia A, Barzaghi C, Garavaglia B, Bergamini C, Fato R, Mora G, Del Bo R, Fortunato F, Borellini L, Trezzi I, Compagnoni GM, Monfrini E, Frattini E, Bonato S, Cogiamanian F, Ardolino G, Priori A, Bresolin N, Corti S, Comi GP, Di Fonzo A. Mutational analysis of COQ2 in patients with MSA in Italy. Neurobiol Aging. 2016;45:213.e1–213.e2.
Schottlaender LV, Houlden H (2014) Multiple-System Atrophy (MSA) Brain bank collaboration. Mutant COQ2 in multiple-system atrophy. N Engl J Med 371(1):81
Sharma M, Wenning G, Krüger R; European multiple-system atrophy study group (EMSA-SG). Mutant COQ2 in multiple-system atrophy. N Engl J Med 2014;371(1):80–81.
Zhao Q, Yang X, Tian S, An R, Zheng J, Xu Y (2016) Association of the COQ2 V393A variant with risk of multiple system atrophy in East Asians: a case-control study and meta-analysis of the literature. Neurol Sci 37(3):423–430
Barca E, Kleiner G, Tang G, Ziosi M, Tadesse S, Masliah E, Louis ED, Faust P et al (2016) Decreased coenzyme Q10 levels in multiple system atrophy cerebellum. J Neuropathol Exp Neurol 75(7):663–672
Schottlaender LV, Bettencourt C, Kiely AP, Chalasani A, Neergheen V, Holton JL, Hargreaves I, Houlden H (2016) Coenzyme Q10 levels are decreased in the cerebellum of multiple-system atrophy patients. PLoS One 11(2):e0149557
Compta Y, Giraldo DM, Muñoz E, Antonelli F, Fernández M, Bravo P, Soto M, Cámara A et al (2018) Catalan MSA Registry (CMSAR). Cerebrospinal fluid levels of coenzyme Q10 are reduced in multiple system atrophy. Parkinsonism Relat Disord 46:16–23
Mitsui J, Matsukawa T, Yasuda T, Ishiura H, Tsuji S (2016) Plasma coenzyme Q10 levels in patients with multiple system atrophy. JAMA Neurol 73(8):977–980
Kasai T, Tokuda T, Ohmichi T, Ishii R, Tatebe H, Nakagawa M, Mizuno T (2016) Serum levels of coenzyme Q10 in patients with multiple system atrophy. PLoS One 11(1):e0147574
Monzio Compagnoni G, Kleiner G, Bordoni A, Fortunato F, Ronchi D, Salani S, Guida M, Corti C et al (2018) Mitochondrial dysfunction in fibroblasts of multiple system atrophy. Biochim Biophys Acta Mol basis Dis 1864(12):3588–3597
Monzio Compagnoni G, Kleiner G, Samarani M, Aureli M, Faustini G, Bellucci A, Ronchi D, Bordoni A et al (2018) Mitochondrial dysregulation and impaired autophagy in iPSC-derived dopaminergic neurons of multiple system atrophy. Stem Cell Reports 11(5):1185–1198
Nakamoto FK, Okamoto S, Mitsui J, Sone T, Ishikawa M, Yamamoto Y, Kanegae Y, Nakatake Y et al (2018) The pathogenesis linked to coenzyme Q10 insufficiency in iPSC-derived neurons from patients with multiple-system atrophy. Sci Rep 8(1):14215
Stefanova N, Reindl M, Neumann M, Haass C, Poewe W, Kahle PJ, Wenning GK (2005) Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am J Pathol 166(3):869–876
Ubhi K, Lee PH, Adame A, Inglis C, Mante M, Rockenstein E, Stefanova N, Wenning GK et al (2009) Mitochondrial inhibitor 3-nitropropionic acid enhances oxidative modification of alpha-synuclein in a transgenic mouse model of multiple system atrophy. J Neurosci Res 87(12):2728–2739
Collier TJ, Kanaan NM, Kordower JH (2011) Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat Rev Neurosci 12(6):359–366
Kirkwood TB (2005) Understanding the odd science of aging. Cell. 120(4):437–447
Jang JY, Blum A, Liu J, Finkel T (2018) The role of mitochondria in aging. J Clin Invest 128(9):3662–3670
Sun N, Youle RJ, Finkel T (2016) The mitochondrial basis of aging. Mol Cell 61(5):654–666
Theurey P, Pizzo P. The Aging Mitochondria. Genes (Basel). 2018;9(1).
Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC (1992) Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet 2(4):324–329
Khaidakov M, Heflich RH, Manjanatha MG, Myers MB, Aidoo A (2003) Accumulation of point mutations in mitochondrial DNA of aging mice. Mutat Res 526(1–2):1–7
Schwarze SR, Lee CM, Chung SS, Roecker EB, Weindruch R, Aiken JM (1995) High levels of mitochondrial DNA deletions in skeletal muscle of old rhesus monkeys. Mech Ageing Dev 83(2):91–101
Ojaimi J, Masters CL, Opeskin K, McKelvie P, Byrne E. Mitochondrial respiratory chain activity in the human brain as a function of age. Mech Ageing Dev 1999;111(1):39–47.
Preston CC, Oberlin AS, Holmuhamedov EL, Gupta A, Sagar S, Syed RH, Siddiqui SA, Raghavakaimal S et al (2008) Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech Ageing Dev 129(6):304–312
Sun N, Yun J, Liu J, Malide D, Liu C, Rovira II, Holmström KM, Fergusson MM et al (2015) Measuring in vivo mitophagy. Mol Cell 60(4):685–696
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11(3):298–300
Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S et al (2004) Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 429(6990):417–423
Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY et al (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 309(5733):481–484
DiMauro S (2004) Mitochondrial diseases. Biochim Biophys Acta 1658(1–2):80–88
Pacelli C, Giguere N, Bourque M, Levesque M, Ruth SS, Trudeau L (2015) Elevated mitochondrial bioenergetics and axonal Arborization size are key contributors to the vulnerability of dopamine neurons. Curr Biol 25:2349–2360
Damiano M, Galvan L, Déglon N, Brouillet E (2010) Mitochondria in Huntington’s disease. Biochim Biophys Acta 1802(1):52–61
Elfawy HA, Das B (2019) Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: etiologies and therapeutic strategies. Life Sci 218:165–184
Wang J, Chen GJ (2016) Mitochondria as a therapeutic target in Alzheimer’s disease. Genes Dis 3(3):220–227
Cenini G, Voos W (2019) Mitochondria as potential targets in Alzheimer disease therapy: an update. Front Pharmacol 10:902
Thomas B, Beal MF (2010) Mitochondrial therapies for Parkinson’s disease. Mov Disord 25(Suppl 1):S155–S160
Parkinson Study Group QE3 Investigators (2014) A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol 71(5):543–552
Acknowledgments
The support from Associazione Amici del Centro Dino Ferrari is gratefully acknowledged.
Funding
The financial support was received from the Fresco Institute for Parkinson’s and Movement Disorders.
Author information
Authors and Affiliations
Contributions
Conceptualization and design: GMC; acquisition and analysis of data: GMC; initial writing of the manuscript: GMC; critical revision of the manuscript for important intellectual content: GMC, ADF, SC, GPC, NB, EM.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Monzio Compagnoni, G., Di Fonzo, A., Corti, S. et al. The Role of Mitochondria in Neurodegenerative Diseases: the Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol Neurobiol 57, 2959–2980 (2020). https://doi.org/10.1007/s12035-020-01926-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01926-1