Abstract
Finding an effective therapeutic approach is a primary goal for current and future research for amyotrophic lateral sclerosis (ALS), a fatal neurological disease characterized by degeneration and loss of upper and lower motor neurons. Transplantation approaches based on stem cells have been attempted in virtue of their potential to contrast simultaneously different ALS pathogenic aspects including either the replacement of lost cells or the protection of motor neurons from degeneration and toxic microenvironment. Here, we critically review the recent translational research aimed at the assessment of stem cell transplantation safety and feasibility in the treatment of ALS. Most of these efforts aim to exert a neuroprotective action rather than cell replacement. Critical aspects that emerge in these studies are the need for the identification of the most effective therapeutic cell source (mesenchymal stem cells, immune, or neural stem cells), the definition of the optimal injection site (cortical area, spinal cord, or muscles) with a suitable administration protocol (local or systemic injection), and the analysis of therapeutic mechanisms, which are necessary steps in order to overcome the hurdles posed by previous in vivo human studies. New perspectives will also be offered by the increasing number of induced pluripotent stem cell-based therapies that are now being tested in clinical trials. A thorough analysis of recently completed trials is the foundation for continued progress in cellular therapy for ALS and other neurodegenerative disorders.


Similar content being viewed by others
References
Hardiman O, Al-Chalabi A, Chio A et al (2017) Amyotrophic lateral sclerosis. Nat Rev Dis Primers 3:17071. https://doi.org/10.1038/nrdp.2017.71
Brown RH, Al-Chalabi A (2017) Amyotrophic lateral sclerosis. N Engl J Med 377:162–172. https://doi.org/10.1056/NEJMra1603471
Miller RG, Mitchell JD, Moore DH (2012) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev:CD001447. https://doi.org/10.1002/14651858.CD001447.pub3
Bucchia M, Ramirez A, Parente V, Simone C, Nizzardo M, Magri F, Dametti S, Corti S (2015) Therapeutic development in amyotrophic lateral sclerosis. Clin Ther 37:668–680. https://doi.org/10.1016/j.clinthera.2014.12.020
Al-Chalabi A, Andersen PM, Chandran S et al (2017) July 2017 ENCALS statement on edaravone. Amyotroph Lateral Scler Frontotemporal Degener 18:471–474. https://doi.org/10.1080/21678421.2017.1369125
Crisafulli SG, Brajkovic S, Cipolat Mis MS, Parente V, Corti S (2018) Therapeutic strategies under development targeting inflammatory mechanisms in amyotrophic lateral sclerosis. Mol Neurobiol 55:2789–2813. https://doi.org/10.1007/s12035-017-0532-4
Faravelli I, Riboldi G, Nizzardo M, Simone C, Zanetta C, Bresolin N, Comi GP, Corti S (2014) Stem cell transplantation for amyotrophic lateral sclerosis: therapeutic potential and perspectives on clinical translation. Cell Mol Life Sci 71:3257–3268. https://doi.org/10.1007/s00018-014-1613-4
Abati E, Bresolin N, Pietro CG, Corti S (2018) Preconditioning and cellular engineering to increase the survival of transplanted neural stem cells for motor neuron disease therapy. Mol Neurobiol. https://doi.org/10.1007/s12035-018-1305-4
Sakowski SA, Schuyler AD, Feldman EL (2009) Insulin-like growth factor-I for the treatment of amyotrophic lateral sclerosis. Amyotroph Lateral Scler 10:63–73. https://doi.org/10.1080/17482960802160370
Javorkova E, Matejckova N, Zajicova A, Hermankova B, Hajkova M, Bohacova P, Kossl J, Krulova M et al (2018) Immunomodulatory properties of bone marrow mesenchymal stem cells from patients with amyotrophic lateral sclerosis and healthy donors. J NeuroImmune Pharmacol. https://doi.org/10.1007/s11481-018-9812-7
Rizzo F, Riboldi G, Salani S, Nizzardo M, Simone C, Corti S, Hedlund E (2014) Cellular therapy to target neuroinflammation in amyotrophic lateral sclerosis. Cell Mol Life Sci 71:999–1015. https://doi.org/10.1007/s00018-013-1480-4
Mazzini L, Ferrari D, Andjus PR, Buzanska L, Cantello R, de Marchi F, Gelati M, Giniatullin R et al (2018) Advances in stem cell therapy for amyotrophic lateral sclerosis. Expert Opin Biol Ther 18:865–881. https://doi.org/10.1080/14712598.2018.1503248
Baloh RH, Glass JD, Svendsen CN (2018) Stem cell transplantation for amyotrophic lateral sclerosis. Curr Opin Neurol 31:655–661. https://doi.org/10.1097/WCO.0000000000000598
Yan J, Xu L, Welsh AM, Hatfield G, Hazel T, Johe K, Koliatsos VE (2007) Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med 4:e39. https://doi.org/10.1371/journal.pmed.0040039
Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatsos VE (2009) Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J Comp Neurol 514:297–309. https://doi.org/10.1002/cne.22022
Corti S, Nizzardo M, Nardini M, Donadoni C, Salani S, del Bo R, Papadimitriou D, Locatelli F et al (2009) Motoneuron transplantation rescues the phenotype of SMARD1 (spinal muscular atrophy with respiratory distress type 1). J Neurosci 29:11761–11771. https://doi.org/10.1523/JNEUROSCI.2734-09.2009
Bonner JF, Blesch A, Neuhuber B, Fischer I (2010) Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res 88:1182–1192. https://doi.org/10.1002/jnr.22288
Silani V, Calzarossa C, Cova L, Ticozzi N (2010) Stem cells in amyotrophic lateral sclerosis: motor neuron protection or replacement? CNS Neurol Disord Drug Targets 9:314–324
Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song SW, Likhite S et al (2011) Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol 29:824–828. https://doi.org/10.1038/nbt.1957
Clement AM, Nguyen MD, Roberts EA et al (2003) Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302:113–117. https://doi.org/10.1126/science.1086071
Lepore AC, O’Donnell J, Kim AS et al (2011) Human glial-restricted progenitor transplantation into cervical spinal cord of the SOD1 mouse model of ALS. PLoS One 6:e25968. https://doi.org/10.1371/journal.pone.0025968
Nicaise C, Mitrecic D, Falnikar A, Lepore AC (2015) Transplantation of stem cell-derived astrocytes for the treatment of amyotrophic lateral sclerosis and spinal cord injury. World J Stem Cells 7:380–398. https://doi.org/10.4252/wjsc.v7.i2.380
Fischer LR, Glass JD (2007) Axonal degeneration in motor neuron disease. Neurodegener Dis 4:431–442. https://doi.org/10.1159/000107704
Kaspar BK, Lladó J, Sherkat N, Rothstein JD, Gage FH (2003) Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 301:839–842. https://doi.org/10.1126/science.1086137
Allodi I, Comley L, Nichterwitz S, Nizzardo M, Simone C, Benitez JA, Cao M, Corti S et al (2016) Differential neuronal vulnerability identifies IGF-2 as a protective factor in ALS. Sci Rep 6:25960. https://doi.org/10.1038/srep25960
Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN (2007) GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One 2:e689. https://doi.org/10.1371/journal.pone.0000689
Li X, Hacker M (2017) Molecular imaging in stem cell-based therapies of cardiac diseases. Adv Drug Deliv Rev 120:71–88. https://doi.org/10.1016/j.addr.2017.07.012
Moshayedi P, Nih LR, Llorente IL, Berg AR, Cinkornpumin J, Lowry WE, Segura T, Carmichael ST (2016) Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 105:145–155. https://doi.org/10.1016/j.biomaterials.2016.07.028
Corti S, Faravelli I, Cardano M, Conti L (2015) Human pluripotent stem cells as tools for neurodegenerative and neurodevelopmental disease modeling and drug discovery. Expert Opin Drug Discovery 10:615–629. https://doi.org/10.1517/17460441.2015.1037737
Sareen D, Gowing G, Sahabian A, Staggenborg K, Paradis R, Avalos P, Latter J, Ornelas L et al (2014) Human induced pluripotent stem cells are a novel source of neural progenitor cells (iNPCs) that migrate and integrate in the rodent spinal cord. J Comp Neurol 522:2707–2728. https://doi.org/10.1002/cne.23578
Nizzardo M, Bucchia M, Ramirez A, Trombetta E, Bresolin N, Comi GP, Corti S (2016) iPSC-derived LewisX+CXCR4+β1-integrin+ neural stem cells improve the amyotrophic lateral sclerosis phenotype by preserving motor neurons and muscle innervation in human and rodent models. Hum Mol Genet 25:3152–3163. https://doi.org/10.1093/hmg/ddw163
Nizzardo M, Simone C, Rizzo F, Ruggieri M, Salani S, Riboldi G, Faravelli I, Zanetta C et al (2014) Minimally invasive transplantation of iPSC-derived ALDHhiSSCloVLA4+ neural stem cells effectively improves the phenotype of an amyotrophic lateral sclerosis model. Hum Mol Genet 23:342–354. https://doi.org/10.1093/hmg/ddt425
Kavyasudha C, Macrin D, ArulJothi KN et al (2018) Clinical applications of induced pluripotent stem cells - Stato Attuale. Adv Exp Med Biol 1079:127–149. https://doi.org/10.1007/5584_2018_173
Mansour AA, Gonçalves JT, Bloyd CW et al (2018) An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol 36:432–441. https://doi.org/10.1038/nbt.4127
Bianco P (2014) “Mesenchymal” stem cells. Annu Rev Cell Dev Biol 30:677–704. https://doi.org/10.1146/annurev-cellbio-100913-013132
Lindner M, Klotz L, Wiendl H (2018) Mechanisms underlying lesion development and lesion distribution in CNS autoimmunity. J Neurochem 146:122–132. https://doi.org/10.1111/jnc.14339
Corti S, Nizzardo M, Nardini M, Donadoni C, Salani S, Simone C, Falcone M, Riboldi G et al (2010) Systemic transplantation of c-kit+ cells exerts a therapeutic effect in a model of amyotrophic lateral sclerosis. Hum Mol Genet 19:3782–3796. https://doi.org/10.1093/hmg/ddq293
Lamanna JJ, Miller JH, Riley JP, Hurtig CV, Boulis NM (2013) Cellular therapeutics delivery to the spinal cord: technical considerations for clinical application. Ther Deliv 4:1397–1410. https://doi.org/10.4155/tde.13.111
Squires A, Oshinski JN, Boulis NM, Tse ZTH (2018) SpinoBot: an MRI-guided needle positioning system for spinal cellular therapeutics. Ann Biomed Eng 46:475–487. https://doi.org/10.1007/s10439-017-1960-z
Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H et al (2001) Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med 344:710–719. https://doi.org/10.1056/NEJM200103083441002
Staff NP, Madigan NN, Morris J et al (2016) Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology 87:2230–2234. https://doi.org/10.1212/WNL.0000000000003359
Robey P (2017) “Mesenchymal stem cells”: fact or fiction, and implications in their therapeutic use. F1000Res 6:524. https://doi.org/10.12688/f1000research.10955.1
Sipp D, Robey PG, Turner L (2018) Clear up this stem-cell mess. Nature 561:455–457. https://doi.org/10.1038/d41586-018-06756-9
Mazzini L, Fagioli F, Boccaletti R, Mareschi K, Oliveri G, Olivieri C, Pastore I, Marasso R et al (2003) Stem cell therapy in amyotrophic lateral sclerosis: a methodological approach in humans. Amyotroph Lateral Scler Other Motor Neuron Disord 4:158–161
Ciervo Y, Ning K, Jun X, Shaw PJ, Mead RJ (2017) Advances, challenges and future directions for stem cell therapy in amyotrophic lateral sclerosis. Mol Neurodegener 12:85. https://doi.org/10.1186/s13024-017-0227-3
Ng F, Boucher S, Koh S, Sastry KSR, Chase L, Lakshmipathy U, Choong C, Yang Z et al (2008) PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 112:295–307. https://doi.org/10.1182/blood-2007-07-103697
Syková E, Rychmach P, Drahorádová I, Konrádová ŠI, Růžičková K, Voříšek I, Forostyak S, Homola A et al (2017) Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIa clinical trial. Cell Transplant 26:647–658. https://doi.org/10.3727/096368916X693716
Oh K-W, Moon C, Kim HY, Oh SI, Park J, Lee JH, Chang IY, Kim KS et al (2015) Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med 4:590–597. https://doi.org/10.5966/sctm.2014-0212
Gothelf Y, Kaspi H, Abramov N, Aricha R (2017) miRNA profiling of NurOwn®: mesenchymal stem cells secreting neurotrophic factors. Stem Cell Res Ther 8:249. https://doi.org/10.1186/s13287-017-0692-1
Petrou P, Gothelf Y, Argov Z, Gotkine M, Levy YS, Kassis I, Vaknin-Dembinsky A, Ben-Hur T et al (2016) Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: results of phase 1/2 and 2a clinical trials. JAMA Neurol 73:337–344. https://doi.org/10.1001/jamaneurol.2015.4321
Daley GQ (2012) The promise and perils of stem cell therapeutics. Cell Stem Cell 10:740–749. https://doi.org/10.1016/j.stem.2012.05.010
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I et al (2017) Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 377:2545–2554. https://doi.org/10.1056/NEJMoa1708566
Corti S, Locatelli F, Donadoni C, Guglieri M, Papadimitriou D, Strazzer S, del Bo R, Comi GP (2004) Wild-type bone marrow cells ameliorate the phenotype of SOD1-G93A ALS mice and contribute to CNS, heart and skeletal muscle tissues. Brain 127:2518–2532. https://doi.org/10.1093/brain/awh273
Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR et al (2006) Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 103:16021–16026. https://doi.org/10.1073/pnas.0607423103
Thonhoff JR, Simpson EP, Appel SH (2018) Neuroinflammatory mechanisms in amyotrophic lateral sclerosis pathogenesis. Curr Opin Neurol 31:635–639. https://doi.org/10.1097/WCO.0000000000000599
Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A et al (2015) Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 7:315ra189–315ra189. https://doi.org/10.1126/scitranslmed.aad4134
Beers DR, Henkel JS, Zhao W, Wang J, Appel SH (2008) CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci 105:15558–15563. https://doi.org/10.1073/pnas.0807419105
Zhao W, Beers DR, Liao B, Henkel JS, Appel SH (2012) Regulatory T lymphocytes from ALS mice suppress microglia and effector T lymphocytes through different cytokine-mediated mechanisms. Neurobiol Dis 48:418–428. https://doi.org/10.1016/j.nbd.2012.07.008
Thonhoff JR, Beers DR, Zhao W, Pleitez M, Simpson EP, Berry JD, Cudkowicz ME, Appel SH (2018) Expanded autologous regulatory T-lymphocyte infusions in ALS: a phase I, first-in-human study. Neurol Neuroimmunol Neuroinflamm 5:e465. https://doi.org/10.1212/NXI.0000000000000465
Mazzini L, Gelati M, Profico DC, Sgaravizzi G, Projetti Pensi M, Muzi G, Ricciolini C, Rota Nodari L et al (2015) Human neural stem cell transplantation in ALS: initial results from a phase I trial. J Transl Med 13:17. https://doi.org/10.1186/s12967-014-0371-2
Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman EL (2012) Lumbar Intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells 30:1144–1151. https://doi.org/10.1002/stem.1079
Feldman EL, Boulis NM, Hur J, Johe K, Rutkove SB, Federici T, Polak M, Bordeau J et al (2014) Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol 75:363–373. https://doi.org/10.1002/ana.24113
Glass JD, Hertzberg VS, Boulis NM, Riley J, Federici T, Polak M, Bordeau J, Fournier C et al (2016) Transplantation of spinal cord–derived neural stem cells for ALS. Neurology 87:392–400. https://doi.org/10.1212/WNL.0000000000002889
Guo X, Johe K, Molnar P, Davis H, Hickman J (2010) Characterization of a human fetal spinal cord stem cell line, NSI-566RSC, and its induction to functional motoneurons. J Tissue Eng Regen Med 4:181–193. https://doi.org/10.1002/term.223
Xu L, Yan J, Chen D, Welsh AM, Hazel T, Johe K, Hatfield G, Koliatsos VE (2006) Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation 82:865–875. https://doi.org/10.1097/01.tp.0000235532.00920.7a
Xu L, Shen P, Hazel T, Johe K, Koliatsos VE (2011) Dual transplantation of human neural stem cells into cervical and lumbar cord ameliorates motor neuron disease in SOD1 transgenic rats. Neurosci Lett 494:222–226. https://doi.org/10.1016/j.neulet.2011.03.017
Fournier CN, Schoenfeld D, Berry JD, Cudkowicz ME, Chan J, Quinn C, Brown RH, Salameh JS et al (2018) An open label study of a novel immunosuppression intervention for the treatment of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 19:242–249. https://doi.org/10.1080/21678421.2017.1421666
Thomsen GM, Avalos P, Ma AA, Alkaslasi M, Cho N, Wyss L, Vit JP, Godoy M et al (2018) Transplantation of neural progenitor cells expressing glial cell line-derived neurotrophic factor into the motor cortex as a strategy to treat amyotrophic lateral sclerosis. Stem Cells 36:1122–1131. https://doi.org/10.1002/stem.2825
Boulis NM, Federici T, Glass JD, Lunn JS, Sakowski SA, Feldman EL (2012) Translational stem cell therapy for amyotrophic lateral sclerosis. Nat Rev Neurol 8:172–176. https://doi.org/10.1038/nrneurol.2011.191
(2018) Voices: crossing the valley of death. Cell Stem Cell 23:639–641. https://doi.org/10.1016/J.STEM.2018.10.021
King NM, Perrin J (2014) Ethical issues in stem cell research and therapy. Stem Cell Res Ther 5:85. https://doi.org/10.1186/scrt474
Henderson GE, Churchill LR, Davis AM, Easter MM, Grady C, Joffe S, Kass N, King NMP et al (2007) Clinical trials and medical care: defining the therapeutic misconception. PLoS Med 4:e324. https://doi.org/10.1371/journal.pmed.0040324
Berkowitz AL, Miller MB, Mir SA, Cagney D, Chavakula V, Guleria I, Aizer A, Ligon KL et al (2016) Glioproliferative lesion of the spinal cord as a complication of “stem-cell tourism”. N Engl J Med 375:196–198. https://doi.org/10.1056/NEJMc1600188
Barker RA, Widner H (2004) Immune problems in central nervous system cell therapy. NeuroRX 1:472–481. https://doi.org/10.1602/neurorx.1.4.472
Srivastava AK, Gross SK, Almad AA, Bulte CA, Maragakis NJ, Bulte JWM (2017) Serial in vivo imaging of transplanted allogeneic neural stem cell survival in a mouse model of amyotrophic lateral sclerosis. Exp Neurol 289:96–102. https://doi.org/10.1016/j.expneurol.2016.12.011
Poesen K, De Schaepdryver M, Stubendorff B et al (2017) Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 88:2302–2309. https://doi.org/10.1212/WNL.0000000000004029
McCombe PA, Pfluger C, Singh P et al (2015) Serial measurements of phosphorylated neurofilament-heavy in the serum of subjects with amyotrophic lateral sclerosis. J Neurol Sci 353:122–129. https://doi.org/10.1016/j.jns.2015.04.032
Escorcio-Bezerra ML, Abrahao A, Nunes KF, de Oliveira Braga NI, Oliveira ASB, Zinman L, Manzano GM (2018) Motor unit number index and neurophysiological index as candidate biomarkers of presymptomatic motor neuron loss in amyotrophic lateral sclerosis. Muscle Nerve 58:204–212. https://doi.org/10.1002/mus.26087
Acknowledgments
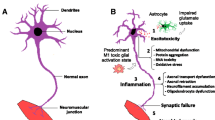
We thank the Associazione Amici del Centro Dino Ferrari for its support. The figure was modified from images from Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License. http://smart.servier.com/
Funding
The following grant supports are gratefully acknowledged: Italian Ministry of Health-RF-2016-02362317 and AFM-Telethon-2015, “Optimized Transplantation of hiPSC-derived LeX+CXCR4+VLA4 neural stem cells as a therapy for SMARD1” to GPC, and CROSS-NEUROD Grant ID: 778003 to SC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abati, E., Bresolin, N., Comi, G. et al. Advances, Challenges, and Perspectives in Translational Stem Cell Therapy for Amyotrophic Lateral Sclerosis. Mol Neurobiol 56, 6703–6715 (2019). https://doi.org/10.1007/s12035-019-1554-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1554-x