Abstract
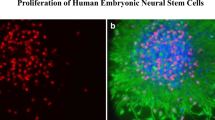
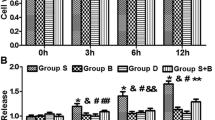
Numerous studies suggest a long duration of anesthesia during the late gestation period and infancy is associated with an increased risk of neuronal damage and neurocognitive impairment. The noble gas xenon is an anesthetic that is reported to have neuroprotective effects in some circumstances at certain concentrations. Currently, the effects of xenon on the brain and its potential neuroprotective properties, and/or the effects of xenon used in combination with other anesthetics, are not clearly understood and some reported data appear contradictory. In the present study, human neural stem cells were employed as a human-relevant model to evaluate the effects of xenon when it was co-administered with propofol, a frequently used anesthetic in pediatric anesthesia, and to understand the mechanism(s). The expression of polysialic acid (PSA) neural cell adhesion molecule (NCAM) on human neural stem cell–differentiated neurons was investigated as a key target molecule. PSA is a specific marker of developing neurons. It is essential for neuronal viability and plasticity. Human neural stem cells were maintained in neural differentiation medium and directed to differentiate into neuronal and glial lineages, and were exposed to propofol (50 μM) for 16 h in the presence or absence of xenon (33%). The neural stem cell–derived neurons were characterized by labelling cells with PSA-NCAM, after 5 days of differentiation. Propofol- and/or xenon-induced neurotoxicities were determined by measuring PSA immunoreactivity. A time course study showed that neuronal cell surface PSA was clearly cleaved off from NCAM by endoneuraminidase N (Endo-N), and eliminated PSA immunostaining was not re-expressed 4, 8, or 16 h after Endo-N washout. However, in the presence of 33% xenon, intense PSA staining on neuronal cell surface and processes was evident 16 h after Endo-N washout. In addition, prolonged (16 h) propofol exposure significantly decreased the positive rate of PSA-labeled neurons. When combined with xenon, propofol’s adverse effects on neurons were attenuated. This work, conducted on the human neural stem cell–derived models, has provided evidence of the beneficiary effects of xenon on neurons and helps develop xenon-based anesthesia regimens in the pediatric population.






Similar content being viewed by others
References
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23(3):876–882
Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA et al (2011) Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol 33(2):220–230. https://doi.org/10.1016/j.ntt.2011.01.001
Slikker W Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC et al (2007) Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci 98(1):145–158. https://doi.org/10.1093/toxsci/kfm084
Bai N, Aida T, Yanagisawa M, Katou S, Sakimura K, Mishina M, Tanaka K (2013) NMDA receptor subunits have different roles in NMDA-induced neurotoxicity in the retina. Mol Brain 6:34. https://doi.org/10.1186/1756-6606-6-34
Liu F, Rainosek SW, Sadovova N, Fogle CM, Patterson TA, Hanig JP, Paule MG, Slikker W Jr et al (2014) Protective effect of acetyl-l-carnitine on propofol-induced toxicity in embryonic neural stem cells. Neurotoxicology 42C:49–57. https://doi.org/10.1016/j.neuro.2014.03.011
Cattano D, Valleggi S, Cavazzana AO, Patel CB, Ma D, Maze M, Giunta F (2011) Xenon exposure in the neonatal rat brain: effects on genes that regulate apoptosis. Minerva Anestesiol 77(6):571–578
Cattano D, Valleggi S, Ma D, Kastsiuchenka O, Abramo A, Sun P, Cavazzana AO, Natale G et al (2008) Xenon induces transcription of ADNP in neonatal rat brain. Neurosci Lett 440(3):217–221. https://doi.org/10.1016/j.neulet.2008.05.086
Ma D, Williamson P, Januszewski A, Nogaro MC, Hossain M, Ong LP, Shu Y, Franks NP et al (2007) Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology 106(4):746–753. https://doi.org/10.1097/01.anes.0000264762.48920.80
Sabir H, Bishop S, Cohen N, Maes E, Liu X, Dingley J, Thoresen M (2013) Neither xenon nor fentanyl induces neuroapoptosis in the newborn pig brain. Anesthesiology 119(2):345–357. https://doi.org/10.1097/ALN.0b013e318294934d
Wilhelm S, Ma D, Maze M, Franks NP (2002) Effects of xenon on in vitro and in vivo models of neuronal injury. Anesthesiology 96(6):1485–1491. https://doi.org/10.1097/00000542-200206000-00031
Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP (2004) Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol 65(2):443–452. https://doi.org/10.1124/mol.65.2.443
Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP (2007) Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology 107(5):756–767. https://doi.org/10.1097/01.anes.0000287061.77674.71
Bantel C, Maze M, Trapp S (2009) Neuronal preconditioning by inhalational anesthetics: evidence for the role of plasmalemmal adenosine triphosphate-sensitive potassium channels. Anesthesiology 110(5):986–995. https://doi.org/10.1097/ALN.0b013e31819dadc7
Rakic P, Cameron RS, Komuro H (1994) Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol 4(1):63–69
Edelman GM (1986) Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol 2:81–116. https://doi.org/10.1146/annurev.cb.02.110186.000501
Finne J, Finne U, Deagostini-Bazin H, Goridis C (1983) Occurrence of alpha 2-8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun 112(2):482–487
Rougon G (1993) Structure, metabolism and cell biology of polysialic acids. Eur J Cell Biol 61(2):197–207
Rutishauser U, Acheson A, Hall AK, Mann DM, Sunshine J (1988) The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science 240(4848):53–57
Doherty P, Cohen J, Walsh FS (1990) Neurite outgrowth in response to transfected N-CAM changes during development and is modulated by polysialic acid. Neuron 5(2):209–219
Zhang H, Miller RH, Rutishauser U (1992) Polysialic acid is required for optimal growth of axons on a neuronal substrate. J Neurosci 12(8):3107–3114
Kiss JZ, Wang C, Olive S, Rougon G, Lang J, Baetens D, Harry D, Pralong WF (1994) Activity-dependent mobilization of the adhesion molecule polysialic NCAM to the cell surface of neurons and endocrine cells. EMBO J 13(22):5284–5292
Wang C, Pralong WF, Schulz MF, Rougon G, Aubry JM, Pagliusi S, Robert A, Kiss JZ (1996) Functional N-methyl-D-aspartate receptors in O-2A glial precursor cells: a critical role in regulating polysialic acid-neural cell adhesion molecule expression and cell migration. J Cell Biol 135(6 Pt 1):1565–1581
Wang C, Sadovova N, Hotchkiss C, Fu X, Scallet AC, Patterson TA, Hanig J, Paule MG et al (2006) Blockade of N-methyl-D-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicol Sci 91(1):192–201. https://doi.org/10.1093/toxsci/kfj144
Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ (1996) PSA-NCAM is required for activity-induced synaptic plasticity. Neuron 17(3):413–422
Liu F, Patterson TA, Sadovova N, Zhang X, Liu S, Zou X, Hanig JP, Paule MG et al (2013) Ketamine-induced neuronal damage and altered N-methyl-D-aspartate receptor function in rat primary forebrain culture. Toxicol Sci 131(2):548–557. https://doi.org/10.1093/toxsci/kfs296
Wang C, Sadovova N, Fu X, Schmued L, Scallet A, Hanig J, Slikker W (2005) The role of the N-methyl-D-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience 132(4):967–977. https://doi.org/10.1016/j.neuroscience.2005.01.053
Creeley C, Dikranian K, Dissen G, Martin L, Olney J, Brambrink A (2013) Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth 110(Suppl 1):i29–i38. https://doi.org/10.1093/bja/aet173
Vutskits L, Gascon E, Kiss JZ (2003) Removal of PSA from NCAM affects the survival of magnocellular vasopressin- and oxytocin-producing neurons in organotypic cultures of the paraventricular nucleus. Eur J Neurosci 17(10):2119–2126
Vutskits L, Gascon E, Zgraggen E, Kiss JZ (2006) The polysialylated neural cell adhesion molecule promotes neurogenesis in vitro. Neurochem Res 31(2):215–225. https://doi.org/10.1007/s11064-005-9021-7
Gascon E, Vutskits L, Jenny B, Durbec P, Kiss JZ (2007) PSA-NCAM in postnatally generated immature neurons of the olfactory bulb: a crucial role in regulating p75 expression and cell survival. Development 134(6):1181–1190. https://doi.org/10.1242/dev.02808
Gascon E, Vutskits L, Kiss JZ (2010) The role of PSA-NCAM in adult neurogenesis. Adv Exp Med Biol 663:127–136. https://doi.org/10.1007/978-1-4419-1170-4_8
Petridis AK, El-Maarouf A, Rutishauser U (2004) Polysialic acid regulates cell contact-dependent neuronal differentiation of progenitor cells from the subventricular zone. Dev Dyn 230(4):675–684. https://doi.org/10.1002/dvdy.20094
Duveau V, Fritschy JM (2010) PSA-NCAM-dependent GDNF signaling limits neurodegeneration and epileptogenesis in temporal lobe epilepsy. Eur J Neurosci 32(1):89–98. https://doi.org/10.1111/j.1460-9568.2010.07272.x
Butler AK, Uryu K, Morehouse V, Rougon G, Chesselet MF (1997) Regulation of the polysialylated form of the neural cell adhesion molecule in the developing striatum: effects of cortical lesions. J Comp Neurol 389(2):289–308
Butler AK, Uryu K, Rougon G, Chesselet MF (1999) N-Methyl-D-aspartate receptor blockade affects polysialylated neural cell adhesion molecule expression and synaptic density during striatal development. Neuroscience 89(4):1169–1181
Becker CG, Artola A, Gerardy-Schahn R, Becker T, Welzl H, Schachner M (1996) The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res 45(2):143–152. https://doi.org/10.1002/(SICI)1097-4547(19960715)45:2<143::AID-JNR6>3.0.CO;2-A
Fox GB, Fichera G, Barry T, O'Connell AW, Gallagher HC, Murphy KJ, Regan CM (2000) Consolidation of passive avoidance learning is associated with transient increases of polysialylated neurons in layer II of the rat medial temporal cortex. J Neurobiol 45(3):135–141
Ronn LC, Berezin V, Bock E (2000) The neural cell adhesion molecule in synaptic plasticity and ageing. Int J Dev Neurosci 18(2–3):193–199
Acheson A, Sunshine JL, Rutishauser U (1991) NCAM polysialic acid can regulate both cell-cell and cell-substrate interactions. J Cell Biol 114(1):143–153
Doherty P, Skaper SD, Moore SE, Leon A, Walsh FS (1992) A developmentally regulated switch in neuronal responsiveness to NCAM and N-cadherin in the rat hippocampus. Development 115(3):885–892
Liu LT, Xu Y, Tang P (2010) Mechanistic insights into xenon inhibition of NMDA receptors from MD simulations. J Phys Chem B 114(27):9010–9016. https://doi.org/10.1021/jp101687j
Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J (2008) Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke 39(4):1307–1313. https://doi.org/10.1161/STROKEAHA.107.499822
Ma D, Wilhelm S, Maze M, Franks NP (2002) Neuroprotective and neurotoxic properties of the ‘inert’ gas, xenon. Br J Anaesth 89(5):739–746
Rylova A, Maze M (2019) Protecting the brain with xenon anesthesia for neurosurgical procedures. J Neurosurg Anesthesiol 31(1):18–29. https://doi.org/10.1097/ANA.0000000000000494
Doherty P, Ashton SV, Moore SE, Walsh FS (1991) Morphoregulatory activities of NCAM and N-cadherin can be accounted for by G protein-dependent activation of L- and N-type neuronal Ca2+ channels. Cell 67(1):21–33
Williams EJ, Doherty P, Turner G, Reid RA, Hemperly JJ, Walsh FS (1992) Calcium influx into neurons can solely account for cell contact-dependent neurite outgrowth stimulated by transfected L1. J Cell Biol 119(4):883–892
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
The information in these materials is not a formal dissemination of information by the FDA and does not represent agency position or policy.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, F., Liu, S., Patterson, T.A. et al. Effects of Xenon-Based Anesthetic Exposure on the Expression Levels of Polysialic Acid Neural Cell Adhesion Molecule (PSA-NCAM) on Human Neural Stem Cell–Derived Neurons. Mol Neurobiol 57, 217–225 (2020). https://doi.org/10.1007/s12035-019-01771-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01771-x