Abstract
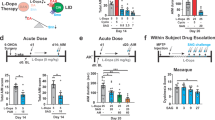
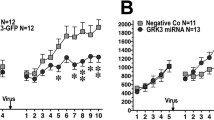
l-DOPA is the main pharmacological therapy for Parkinson’s disease. However, long-term exposure to l-DOPA induces involuntary movements termed dyskinesia. Clinical trials show that dyskinesia is attenuated by metabotropic glutamate receptor type 5 (mGluR5) antagonists. Further, the onset of dyskinesia is delayed by nicotine and mGluR5 expression is lower in smokers than in non-smokers. However, the mechanisms by which mGluR5 modulates dyskinesia and how mGluR5 and nicotine interact have not been established. To address these issues, we studied the role of mGluR5 in D1R-containing neurons in dyskinesia and examined whether nicotine reduces dyskinesia via mGluR5. In the aphakia mouse model of Parkinson’s disease, we selectively knocked down mGluR5 in D1R-containing neurons (aphakia-mGluR5KD-D1). We found that genetic downregulation of mGluR5 decreased dyskinesia in aphakia mice. Although chronic nicotine increased the therapeutic effect of l-DOPA in both aphakia and aphakia-mGluR5KD-D1 mice, it caused a robust reduction in dyskinesia only in aphakia, and not in aphakia-mGluR5KD-D1 mice. Downregulating mGluR5 or nicotine treatment after l-DOPA decreased ERK and histone 3 activation, and FosB expression. Combining nicotine and mGluR5 knockdown did not have an added antidyskinetic effect, indicating that the effect of nicotine might be mediated by downregulation of mGluR5 expression. Treatment of aphakia-mGluR5KD-D1 mice with a negative allosteric modulator did not further modify dyskinesia, suggesting that mGluR5 in non-D1R-containing neurons does not play a role in its development. In conclusion, this work suggests that mGluR5 antagonists reduce dyskinesia by mainly affecting D1R-containing neurons and that the effect of nicotine on dyskinetic signs in aphakia mice is likely via mGluR5.






Similar content being viewed by others
References
Cotzias GC, Van Woert MH, Schiffer LM (1967) Aromatic amino acids and modification of Parkinsonism. N Engl J Med 276:374–379. https://doi.org/10.1056/NEJM196702162760703
De Deurwaerdère P, Di Giovanni G, Millan MJ (2017) Expanding the repertoire of L-DOPA’s actions: a comprehensive review of its functional neurochemistry. Prog Neurobiol 151:57–100. https://doi.org/10.1016/j.pneurobio.2016.07.002
García-Montes JR, Boronat-García A, López-Colomé AM et al (2012) Is nicotine protective against Parkinson’s disease? An experimental analysis. CNS Neurol Disord Drug Targets 11:897–906
Heumann R, Moratalla R, Herrero MT, Chakrabarty K, Drucker-Colín R, Garcia-Montes JR, Simola N, Morelli M (2014) Dyskinesia in Parkinson’s disease: mechanisms and current non-pharmacological interventions. J Neurochem 130:472–489. https://doi.org/10.1111/jnc.12751
Solís O, Moratalla R (2018) Dopamine receptors: homomeric and heteromeric complexes in L-DOPA-induced dyskinesia. J Neural Transm 125:1187–1194. https://doi.org/10.1007/s00702-018-1852-x
Rascol O, Nutt JG, Blin O, Goetz CG, Trugman JM, Soubrouillard C, Carter JH, Currie LJ et al (2001) Induction by dopamine D1 receptor agonist ABT-431 of dyskinesia similar to levodopa in patients with Parkinson disease. Arch Neurol 58:249–254
Guigoni C, Doudnikoff E, Li Q, Bloch B, Bezard E (2007) Altered D(1) dopamine receptor trafficking in parkinsonian and dyskinetic non-human primates. Neurobiol Dis 26:452–463. https://doi.org/10.1016/j.nbd.2007.02.001
Darmopil S, Martín AB, De Diego IR et al (2009) Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol Psychiatry 66:603–613. https://doi.org/10.1016/j.biopsych.2009.04.025
Lindgren HS, Rylander D, Iderberg H et al (2011) Putaminal upregulation of FosB/ΔFosB-like immunoreactivity in Parkinson’s disease patients with dyskinesia. J Parkinsons Dis 1:347–357. https://doi.org/10.3233/JPD-2011-11068
Espadas I, Darmopil S, Vergaño-Vera E, Ortiz O, Oliva I, Vicario-Abejón C, Martín ED, Moratalla R (2012) L-DOPA-induced increase in TH-immunoreactive striatal neurons in parkinsonian mice: insights into regulation and function. Neurobiol Dis 48:271–281. https://doi.org/10.1016/j.nbd.2012.07.012
Palafox-Sánchez V, Mendieta L, Ramírez-García G, Candalija A, Aguilera J, Limón ID (2016) Effect of the C-terminal domain of the heavy chain of tetanus toxin on dyskinesia caused by levodopa in 6-hydroxydopamine-lesioned rats. Pharmacol Biochem Behav 145:33–44. https://doi.org/10.1016/j.pbb.2016.04.001
Solís O, García-Montes JR, Garcia-Sanz P, Herranz AS, Asensio MJ, Kang G, Hiroi N, Moratalla R (2017) Human COMT over-expression confers a heightened susceptibility to dyskinesia in mice. Neurobiol Dis 102:133–139. https://doi.org/10.1016/j.nbd.2017.03.006
Solís O, Garcia-Montes JR, González-Granillo A, Xu M, Moratalla R (2017) Dopamine D3 receptor modulates l-DOPA-induced dyskinesia by targeting D1 receptor-mediated striatal signaling. Cereb Cortex 27:435–446. https://doi.org/10.1093/cercor/bhv231
Ruiz-DeDiego I, Mellstrom B, Vallejo M, Naranjo JR, Moratalla R (2015) Activation of DREAM (downstream regulatory element antagonistic modulator), a calcium-binding protein, reduces L-DOPA-induced dyskinesias in mice. Biol Psychiatry 77:95–105. https://doi.org/10.1016/j.biopsych.2014.03.023
Fieblinger T, Sebastianutto I, Alcacer C, Bimpisidis Z, Maslava N, Sandberg S, Engblom D, Cenci MA (2014) Mechanisms of dopamine D1 receptor-mediated ERK1/2 activation in the parkinsonian striatum and their modulation by metabotropic glutamate receptor type 5. J Neurosci 34:4728–4740. https://doi.org/10.1523/JNEUROSCI.2702-13.2014
Levine A, Huang Y, Drisaldi B, Griffin EA, Pollak DD, Xu S, Yin D, Schaffran C et al (2011) Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med 3:107ra109. https://doi.org/10.1126/scitranslmed.3003062
Calabresi P, Mercuri NB, Sancesario G, Bernardi G (1993) Electrophysiology of dopamine-denervated striatal neurons. Implications for Parkinson’s disease. Brain 116(Pt 2):433–452
Meshul CK, Kamel D, Moore C, Kay TS, Krentz L (2002) Nicotine alters striatal glutamate function and decreases the apomorphine-induced contralateral rotations in 6-OHDA-lesioned rats. Exp Neurol 175:257–274. https://doi.org/10.1006/exnr.2002.7900
Suarez LM, Solis O, Aguado C, Lujan R, Moratalla R (2016) L-DOPA oppositely regulates synaptic strength and spine morphology in D1 and D2 striatal projection neurons in dyskinesia. Cereb Cortex 26:4253–4264. https://doi.org/10.1093/cercor/bhw263
Solís O, García-Sanz P, Herranz AS, Asensio MJ, Moratalla R (2016) L-DOPA reverses the increased free amino acids tissue levels induced by dopamine depletion and rises GABA and tyrosine in the striatum. Neurotox Res 30:67–75. https://doi.org/10.1007/s12640-016-9612-x
Oueslati A, Breysse N, Amalric M, Kerkerian-le Goff L, Salin P (2005) Dysfunction of the cortico-basal ganglia-cortical loop in a rat model of early Parkinsonism is reversed by metabotropic glutamate receptor 5 antagonism. Eur J Neurosci 22:2765–2774. https://doi.org/10.1111/j.1460-9568.2005.04498.x
Testa CM, Standaert DG, Landwehrmeyer GB, Penney JB, Young AB (1995) Differential expression of mGluR5 metabotropic glutamate receptor mRNA by rat striatal neurons. J Comp Neurol 354:241–252. https://doi.org/10.1002/cne.903540207
Conn PJ, Battaglia G, Marino MJ, Nicoletti F (2005) Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci 6:787–798. https://doi.org/10.1038/nrn1763
Bezard E, Pioli EY, Li Q, Girard F, Mutel V, Keywood C, Tison F, Rascol O et al (2014) The mGluR5 negative allosteric modulator dipraglurant reduces dyskinesia in the MPTP macaque model. Mov Disord 29:1074–1079. https://doi.org/10.1002/mds.25920
Grégoire L, Morin N, Ouattara B, Gasparini F, Bilbe G, Johns D, Vranesic I, Sahasranaman S et al (2011) The acute antiparkinsonian and antidyskinetic effect of AFQ056, a novel metabotropic glutamate receptor type 5 antagonist, in L-Dopa-treated parkinsonian monkeys. Parkinsonism Relat Disord 17:270–276. https://doi.org/10.1016/j.parkreldis.2011.01.008
Levandis G, Bazzini E, Armentero MT, Nappi G, Blandini F (2008) Systemic administration of an mGluR5 antagonist, but not unilateral subthalamic lesion, counteracts l-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neurobiol Dis 29:161–168. https://doi.org/10.1016/j.nbd.2007.08.011
Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA (2007) Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J Neurochem 101:483–497. https://doi.org/10.1111/j.1471-4159.2007.04456.x
Ouattara B, Grégoire L, Morissette M, Gasparini F, Vranesic I, Bilbe G, Johns DR, Rajput A et al (2011) Metabotropic glutamate receptor type 5 in levodopa-induced motor complications. Neurobiol Aging 32:1286–1295. https://doi.org/10.1016/j.neurobiolaging.2009.07.014
Vranesic I, Ofner S, Flor PJ, Bilbe G, Bouhelal R, Enz A, Desrayaud S, McAllister K et al (2014) AFQ056/mavoglurant, a novel clinically effective mGluR5 antagonist: identification, SAR and pharmacological characterization. Bioorg Med Chem 22:5790–5803. https://doi.org/10.1016/j.bmc.2014.09.033
Crabbé M, Van der Perren A, Weerasekera A et al (2018) Altered mGluR5 binding potential and glutamine concentration in the 6-OHDA rat model of acute Parkinson’s disease and levodopa-induced dyskinesia. Neurobiol Aging 61:82–92. https://doi.org/10.1016/j.neurobiolaging.2017.09.006
Tison F, Keywood C, Wakefield M, Durif F, Corvol JC, Eggert K, Lew M, Isaacson S et al (2016) A phase 2A trial of the novel mGluR5-negative allosteric modulator dipraglurant for levodopa-induced dyskinesia in Parkinson’s disease. Mov Disord 31:1373–1380. https://doi.org/10.1002/mds.26659
Trenkwalder C, Stocchi F, Poewe W, Dronamraju N, Kenney C, Shah A, von Raison F, Graf A (2016) Mavoglurant in Parkinson’s patients with l-Dopa-induced dyskinesias: two randomized phase 2 studies. Mov Disord 31:1054–1058. https://doi.org/10.1002/mds.26585
Du JJ, Chen SD (2017) Current nondopaminergic therapeutic options for motor symptoms of Parkinson’s disease. Chin Med J 130:1856–1866. https://doi.org/10.4103/0366-6999.211555
De Reuck J, De Weweire M, Van Maele G, Santens P (2005) Comparison of age of onset and development of motor complications between smokers and non-smokers in Parkinson’s disease. J Neurol Sci 231:35–39. https://doi.org/10.1016/j.jns.2004.12.003
Bordia T, Campos C, Huang L, Quik M (2008) Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther 327:239–247. https://doi.org/10.1124/jpet.108.140897
Huang LZ, Grady SR, Quik M (2011) Nicotine reduces L-DOPA-induced dyskinesias by acting at beta2* nicotinic receptors. J Pharmacol Exp Ther 338:932–941. https://doi.org/10.1124/jpet.111.182949
Quik M, Campos C, Grady SR (2013) Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias: studies with parkinsonian nicotinic receptor knockout mice. Biochem Pharmacol 86:1153–1162. https://doi.org/10.1016/j.bcp.2013.06.027
Quik M, Mallela A, Ly J, Zhang D (2013) Nicotine reduces established levodopa-induced dyskinesias in a monkey model of Parkinson’s disease. Mov Disord 28:1398–1406. https://doi.org/10.1002/mds.25594
Quik M, Zhang D, Perez XA, Bordia T (2014) Role for the nicotinic cholinergic system in movement disorders; therapeutic implications. Pharmacol Ther 144:50–59. https://doi.org/10.1016/j.pharmthera.2014.05.004
Quik M, Cox H, Parameswaran N, O’Leary K, Langston JW, di Monte D (2007) Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Ann Neurol 62:588–596. https://doi.org/10.1002/ana.21203
Morissette M, Morin N, Grégoire L, Rajput A, Rajput AH, di Paolo T (2016) Brain α7 nicotinic acetylcholine receptors in MPTP-lesioned monkeys and parkinsonian patients. Biochem Pharmacol 109:62–69. https://doi.org/10.1016/j.bcp.2016.03.023
Akkus F, Ametamey SM, Treyer V, Burger C, Johayem A, Umbricht D, Gomez Mancilla B, Sovago J et al (2013) Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proc Natl Acad Sci U S A 110:737–742. https://doi.org/10.1073/pnas.1210984110
de Aceves Buendia JJ, Tiroshi L, Chiu WH, Goldberg JA (2017) Selective remodeling of glutamatergic transmission to striatal cholinergic interneurons after dopamine depletion. Eur J Neurosci. https://doi.org/10.1111/ejn.13715
Dineley KT, Pandya AA, Yakel JL (2015) Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci 36:96–108. https://doi.org/10.1016/j.tips.2014.12.002
Novak M, Halbout B, O’Connor EC et al (2010) Incentive learning underlying cocaine-seeking requires mGluR5 receptors located on dopamine D1 receptor-expressing neurons. J Neurosci 30:11973–11982. https://doi.org/10.1523/JNEUROSCI.2550-10.2010
Suarez LM, Alberquilla S, García-Montes JR, Moratalla R (2018) Differential synaptic remodeling by dopamine in direct and indirect striatal projection neurons in Pitx3−/− mice, a genetic model of Parkinson’s disease. J Neurosci 38:3619–3630. https://doi.org/10.1523/JNEUROSCI.3184-17.2018
Solís O, Espadas I, Del-Bel EA, Moratalla R (2015) Nitric oxide synthase inhibition decreases l-DOPA-induced dyskinesia and the expression of striatal molecular markers in Pitx3(−/−) aphakia mice. Neurobiol Dis 73:49–59. https://doi.org/10.1016/j.nbd.2014.09.010
Ding Y, Restrepo J, Won L, Hwang DY, Kim KS, Kang UJ (2007) Chronic 3,4-dihydroxyphenylalanine treatment induces dyskinesia in aphakia mice, a novel genetic model of Parkinson’s disease. Neurobiol Dis 27:11–23. https://doi.org/10.1016/j.nbd.2007.03.013
Ding Y, Won L, Britt JP, Lim SAO, McGehee DS, Kang UJ (2011) Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc Natl Acad Sci U S A 108:840–845. https://doi.org/10.1073/pnas.1006511108
Hwang D-Y, Fleming SM, Ardayfio P et al (2005) 3,4-dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: behavioral characterization of a novel genetic model of Parkinson’s disease. J Neurosci 25:2132–2137. https://doi.org/10.1523/JNEUROSCI.3718-04.2005
Collins TJ (2007) ImageJ for microscopy. BioTechniques 43:25–30. https://doi.org/10.2144/000112517
García-Sanz P, Orgaz L, Bueno-Gil G, Espadas I, Rodríguez-Traver E, Kulisevsky J, Gutierrez A, Dávila JC et al (2017) N370S-GBA1 mutation causes lysosomal cholesterol accumulation in Parkinson’s disease. Mov Disord 32:1409–1422. https://doi.org/10.1002/mds.27119
Quik M, Campos C, Bordia T, Strachan JP, Zhang J, McIntosh JM, Letchworth S, Jordan K (2013) α4β2 nicotinic receptors play a role in the nAChR-mediated decline in L-dopa-induced dyskinesias in parkinsonian rats. Neuropharmacology 71:191–203. https://doi.org/10.1016/j.neuropharm.2013.03.038
Quik M, Park KM, Hrachova M, Mallela A, Huang LZ, McIntosh JM, Grady SR (2012) Role for α6 nicotinic receptors in l-dopa-induced dyskinesias in parkinsonian mice. Neuropharmacology 63:450–459. https://doi.org/10.1016/j.neuropharm.2012.04.029
Zhang L, Balan G, Barreiro G, Boscoe BP, Chenard LK, Cianfrogna J, Claffey MM, Chen L et al (2014) Discovery and preclinical characterization of 1-methyl-3-(4-methylpyridin-3-yl)-6-(pyridin-2-ylmethoxy)-1H-pyrazolo-[3,4-b]pyrazine (PF470): a highly potent, selective, and efficacious metabotropic glutamate receptor 5 (mGluR5) negative allosteric modulator. J Med Chem 57:861–877. https://doi.org/10.1021/jm401622k
Domenici MR, Potenza RL, Martire A, Coccurello R, Pèzzola A, Reggio R, Tebano MT, Popoli P (2005) Chronic treatment with the mGlu5R antagonist MPEP reduces the functional effects of the mGlu5R agonist CHPG in the striatum of 6-hydroxydopamine-lesioned rats: possible relevance to the effects of mGlu5R blockade in Parkinson’s disease. J Neurosci Res 80:646–654. https://doi.org/10.1002/jnr.20489
Ouattara B, Gasparini F, Morissette M, Grégoire L, Samadi P, Gomez-Mancilla B, di Paolo T (2010) Effect of L-Dopa on metabotropic glutamate receptor 5 in the brain of parkinsonian monkeys. J Neurochem 113:715–724. https://doi.org/10.1111/j.1471-4159.2010.06635.x
Rascol O, Fox S, Gasparini F, Kenney C, di Paolo T, Gomez-Mancilla B (2014) Use of metabotropic glutamate 5-receptor antagonists for treatment of levodopa-induced dyskinesias. Parkinsonism Relat Disord 20:947–956. https://doi.org/10.1016/j.parkreldis.2014.05.003
Sebastianutto I, Cenci MA (2018) mGlu receptors in the treatment of Parkinson’s disease and L-DOPA-induced dyskinesia. Curr Opin Pharmacol 38:81–89. https://doi.org/10.1016/j.coph.2018.03.003
Pavón N, Martín AB, Mendialdua A, Moratalla R (2006) ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry 59:64–74. https://doi.org/10.1016/j.biopsych.2005.05.044
Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA (2007) Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry 62:800–810. https://doi.org/10.1016/j.biopsych.2006.11.032
Suárez LM, Solís O, Caramés JM, Taravini IR, Solís JM, Murer MG, Moratalla R (2014) L-DOPA treatment selectively restores spine density in dopamine receptor D2-expressing projection neurons in dyskinetic mice. Biol Psychiatry 75:711–722. https://doi.org/10.1016/j.biopsych.2013.05.006
Jong Y-JI, Kumar V, O’Malley KL (2009) Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts. J Biol Chem 284:35827–35838. https://doi.org/10.1074/jbc.M109.046276
Rylander D, Iderberg H, Li Q, Dekundy A, Zhang J, Li H, Baishen R, Danysz W et al (2010) A mGluR5 antagonist under clinical development improves L-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol Dis 39:352–361. https://doi.org/10.1016/j.nbd.2010.05.001
Akkus F, Treyer V, Johayem A, Ametamey SM, Mancilla BG, Sovago J, Buck A, Hasler G (2016) Association of long-term nicotine abstinence with normal metabotropic glutamate receptor-5 binding. Biol Psychiatry 79:474–480. https://doi.org/10.1016/j.biopsych.2015.02.027
Shimohama S, Akaike A, Kimura J (1996) Nicotine-induced protection against glutamate cytotoxicity. Nicotinic cholinergic receptor-mediated inhibition of nitric oxide formation. Ann N Y Acad Sci 777:356–361
Tonnessen BH, Severson SR, Hurt RD, Miller VM (2000) Modulation of nitric-oxide synthase by nicotine. J Pharmacol Exp Ther 295:601–606
Weruaga E, Balkan B, Koylu EO, Pogun S, Alonso JR (2002) Effects of chronic nicotine administration on nitric oxide synthase expression and activity in rat brain. J Neurosci Res 67:689–697. https://doi.org/10.1002/jnr.10158
Giorgi M, D’Angelo V, Esposito Z, Nuccetelli V, Sorge R, Martorana A, Stefani A, Bernardi G et al (2008) Lowered cAMP and cGMP signalling in the brain during levodopa-induced dyskinesias in hemiparkinsonian rats: new aspects in the pathogenetic mechanisms. Eur J Neurosci 28:941–950. https://doi.org/10.1111/j.1460-9568.2008.06387.x
Picconi B, Bagetta V, Ghiglieri V, Paillè V, di Filippo M, Pendolino V, Tozzi A, Giampà C et al (2011) Inhibition of phosphodiesterases rescues striatal long-term depression and reduces levodopa-induced dyskinesia. Brain 134:375–387. https://doi.org/10.1093/brain/awq342
Acknowledgements
In memoriam of Dr. René Drucker-Colín. JR acknowledges the Secretaría de Ciencia Tecnología e Innovación in México City, for fellowship and support. We acknowledge the priceless help of our expert technicians E. Rubio and B. Pro. We thank P. Garcia-Sanz, H. Escobar, S. Alberquilla, and N. Granado for their kind opinion and recommendations.
Funding
This work was supported by grants from the Spanish Ministerios de Economía y Competitividad and of Sanidad Política Social e Igualdad, ISCIII: SAF2016-78207-R, CIBERNED ref. CB06/05/0055, PNSD 2016/33, and PCIN 2015-098; and Foundation Ramon Areces and Secretaria de Ciencia Tecnología e Innovación de la Ciudad de Mexico (037-2016) to RM.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All experiments were conducted in accordance with the guidelines of the European Union Council Directive (86/609/EEC and 2003/65/CE). The protocol was approved by the Spanish Council ethics committee and the committee of ethics and animal experimentation of the Instituto Cajal (CSIC).
Rights and permissions
About this article
Cite this article
García-Montes, JR., Solís, O., Enríquez-Traba, J. et al. Genetic Knockdown of mGluR5 in Striatal D1R-Containing Neurons Attenuates l-DOPA-Induced Dyskinesia in Aphakia Mice. Mol Neurobiol 56, 4037–4050 (2019). https://doi.org/10.1007/s12035-018-1356-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1356-6