Abstract
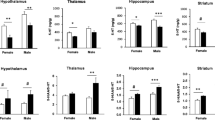
Cystathionine beta synthase (CBS) is one of the 225 genes on chromosome 21 (HSA 21) that are triplicated in persons with trisomy 21 (Down syndrome). Although most triplicate HSA21 genes have their orthologous genes on murine chromosome 16, the murine ortholog of hCBS is on murine chromosome 17 and thus is not present in the well-studied Ts65Dn mouse model of trisomy 21. Persons with trisomy 21 (T21) present deficits in neurotransmission and exhibit early brain aging that can partially be explained by monoamine neurotransmitter alterations. We used transgenic mice for the hCBS gene, which overexpress the CBS protein in various brain regions, to study if CBS overexpression induces modifications in the monoamine neurotransmitters in the hypothalamus, thalamus, hippocampus, and striatum from transgenic and control female and male mice aged 3–4 months and 11–12 months. Sex, age, and brain area each influenced neurotransmitter levels. Briefly, the serotonin pathway was modified by CBS overexpression in various brain areas in female mice but not in male mice. The dopamine pathway was modified in brain regions according to sex and age. These results may allow us to better understand the role of the transsulfuration pathway and especially CBS overexpression in the metabolism of biogenic amines and the catecholamine catabolism in persons with trisomy 21.








Similar content being viewed by others
References
Pueschel SM (1990) Clinical aspects of Down syndrome from infancy to adulthood. Am J Med Genet Suppl 7:52–56
Vicari S (2006) Motor development and neuropsychological patterns in persons with Down syndrome. Behav Genet 36:365–364
Suetsugu M, Mehraein P (1980) Spines distribution along the apical dendrites of the pyramidal neurons in Down’s syndrome. Quant Golgi study Acta Neuropathol 50:207–210
Coyle JT, Oster-GraniteML GJD (1986) The neurobiologic consequences of down syndrome. Brain Res Bull 16:773–787
Lott IT (2012) Neurological phenotypes for Down syndrome across the life span. Prog Brain Res 197:101–121
Hartley D, Blumenthal T, Carrillo M, DiPaolo G, Esralew L, Gardiner K, Granholm AC, Iqbal K et al (2015) Down syndrome and Alzheimer’s disease: common pathways, common goals. Alzheimers Dement 11:700–709
Brodie BB, Shore PA (1957) A concept for a role of serotonin and norepinephrine as chemical mediators in the brain. Ann N YAcad Sci 66:631–642
Eisenhofer G, Kopin IJ, Goldstein DS (2004) Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56:331–349
Kehagia AA, Murray GK, Robbins TW (2010) Learning and cognitive flexibility: fronto-striatal function and monoaminergic modulation. Curr Opin Neurobiol 20:199–20410.
Snyder SH (2011) What dopamine does in the brain. PNAS 108:18869–18871
Tritsch NX, Sabatini BL (2012) Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76:33–50
Cools R, Nakamura K, Daw ND (2011) Serotonin and dopamine unifying affective, activational and decision functions. Neuropsychopharmacol Rev 36:98–113
Xing B, Li YC, Gao WJ (2016) Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res 1641:217–233
Tu JH, Zellweger H (1965) Blood-serotonin deficiency in Down’s syndrome. Lancet 2:715–716
Boullin DJ, O’Brien RA (1971) Abnormalities of 5-Hydroxytryptamine uptake and binding by blood platelets from children with Down’s syndrome. J Physiol 212:287–297
Lott IT, Chase TN, Murphy DL (1972) Down’s syndrome: transport, storage, and metabolism of serotonin in blood platelets. Pediatr Res 6:730–735
Wetterberg L, Gustavson KH, Backström M, Ross SB, Fröden O (1972) Low dopamine-beta-hydroxylase activity in Down’s syndrome. Clin Genet 3:152–153
Coppus AW, Fekkes D, Verhoeven WMA, Tuinier S, Egger JIM et al (2007) Plasma amino acids and neopterin in healthy persons with Down’s syndrome. J Neural Transm 11:1041–1045
Dekker AD, Coppus AM, Vermeiren Y, Aerts T, van Duijn CM, Kremer BP, Naudé PJ, Van Dam D et al (2015) Serum MHPG strongly predicts conversion to Alzheimer’s disease in behaviorally characterized subjects with Down syndrome. J Alzheimers Dis 43(3):871–891
Mann DM, Lincoln J, Yates PO, Brennan CM (1980) Monoamine metabolism in Down syndrome. Lancet 2:1366–1367
Kay AD, Schapiro MB, Riker AK, Haxby JV, Rapoport SI, Cutler NR (1987) Cerebrospinal fluid monoaminergic metabolites are elevated in adults with Down’s syndrome. Ann Neurol 21(4):408–411
Schapiro MB, Kay AD, May C, Ryker AK, Haxby JV, Kaufman S, Milstien S, Rapoport SI (1987) Cerebrospinal fluid monoamines in Down’s syndrome adults at different ages. J Ment Defic Res 31(Pt 3):259–269
Yates CM, Simpson J, Gordon A (1986) Regional brain 5-hydroxytryptamine levels are reduced in senile Down's syndrome as in Alzheimer’s disease. NeuroscLetter 65(2):189–192
Risser D, Lubec G, Cairns N, Herrera-Marschitz M (1997) Excitatory amino acids and monoamines in parahippocampal gyrus and frontal cortical pole of adults with Down syndrome. Life Sci 60:1231–1237
Seidl R, Kaehler ST, Prast H et al (1999) Serotonin (5-HT) in brains of adult patients with Down syndrome. J Neural Trans Sup 57:221–232
Whittle N, Sartori SB, Dierssen M, Lubec G, Singewald N (2007) Fetal Down syndrome brains exhibit aberrant levels of neurotransmitters critical for normal brain development. Pediatrics 120:e1465–e1471
Antonarakis SE, Lyle R, Dermitzakis ET, Reymond A, Deutsch S (2004) Chromosome 21 and Down syndrome: from genomics to pathophysiology. Nat Rev Genet 5:725–738
Gardiner K, Herault Y, Lott IT, Antonarakis SE, Reeves RH, Dierssen M (2010) Down syndrome: from understanding the neurobiology to therapy. J Neurosci 3:14943–14945
Cox DR, Smith SA, Epstein LB, Epstein CJ (1984) Mouse trisomy 16 as an animal model of human trisomy 21 (Down syndrome): production of viable trisomy 16 diploid mouse chimeras. Dev Biol 101(2):416–424
Davisson MT, Schmidt C, Akeson EC (1990) Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog Clin Biol Res 360:263–280
Dierssen M (2012) Down syndrome: the brain in trisomic mode. Nat Rev Neurosci 13(12):844–858
Rueda N, Flórez J, Martínez-Cué C (2012) Mouse models of Down syndrome as a tool to unravel the causes of mental disabilities. Neural Plast 584071
Gotti S, Caricati E, Panzica G (2011) Alterations of brain circuits in Down syndrome murine models. J Chem Neuroanat 42(4):317–326. https://doi.org/10.1016/j.jchemneu.2011.09.002
Das D, Phillips C, Hsieh W, Sumanth K, Dang V, Salehi A (2014) Neurotransmitter-based strategies for the treatment of cognitive dysfunction in Down syndrome. Prog Neuro-Psychopharmacol Biol Psychiatry 54:140–148
Dekker AD, Vermeiren Y, Albac C, Lana-Elola E, Watson-Scales S, Gibbins D, Aerts T, Van Dam D et al (2017) Aging rather than aneuploidy affects monoamine neurotransmitters in brain regions of Down syndrome mouse models. Neurobiol Dis 105:235–244. https://doi.org/10.1016/j.nbd.2017.06.007
Zhang L, Meng K, Jiang X, Liu C, Pao A, Belichenko PV, Kleschevnikov AM, Josselyn S et al (2014) Human chromosome 21 orthologous region on mouse chromosome 17 is a major determinant of Down syndrome-related developmental cognitive deficits. Hum Mol Genet 1; 23(3):578–589
London J, Rouch C, Bui LC, Assayag E, Souchet B, Daubigney F, Medjaoui H, Luquet S et al (2017) Overexpression of the DYRK1A gene (dual-specificity tyrosine phosphorylation-regulated kinase 1A) induces alterations of the serotoninergic and dopaminergic processing in murine brain tissues. Mol Neurobiol 55(5):3822–3831. https://doi.org/10.1007/s12035-017-0591-6
Pereira PL, Magnol L, Sahún I, Brault V, Duchon A, Prandini P, Gruart A, Bizot JC et al (2009) A new mouse model for the trisomy of the Abcg1-U2af1 region reveals the complexity of the combinatorial genetic code of down syndrome. Hum Mol Genet 18(24):4756–4769. https://doi.org/10.1093/hmg/ddp438
Münke M, Kraus JP, Ohura T, Francke U (1988) The gene for cystathionine beta-synthase (CBS) maps to the subtelomeric region on human chromosome 21q and to proximal mouse chromosome 17. Am J Hum Genet 42(4):550–559
Mudd SH, Finkelstein JD, Irreverre F, Laster L (1965) Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J Biol Chem 240:4382–4392
Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16:1066–1071
Chen X, Jhee KH, Kruger WD (2004) Production of the neuromodulator H2S by cystathionine beta synthase via condensation of cysteines and homocysteine. J Biol Chem 279(50):52082–52086
Qu K, Lee SW, Bian JS, Low CM, Wong PTH (2008) Hydrogen sulfide: neurochemistry and neurobiology. Neurochem Int 52:155–165
Yadav PK, Xie P, Banerjee R (2012) Allosteric communication between the pyridoxal 5′-phosphate (PLP) and heme sites in the H2S generator human cystathionine β-synthase. J Biol Chem 287:37611–37620
Chadefaux B, Rethore MO, Raoul O, Ceballos I, Poissonnier M et al (1985) Cystathionine beta synthase: gene dosage effect in trisomy 21. Biochem Biophys Res Com 128:40–44
Pogribna M, Melnyk S, Pogribny I, Chango A, Yi P et al (2001) Homocysteine metabolism in children with Down syndrome: in vitro modulation. Am J Genet 69:88–95
Ichinohe A, Kanaumi T, Takashima S, Enokido Y, Nagai Y, Kimura H (2005) Cystathionine beta-synthase is enriched in the brains of Down’s patients. Biochem Biophys Res Commun 338:1547–1550
Lockstone HE, Harris LW, Swatton JE, Wayland MT, Holland AJ, Bahn S (2007) Gene expression profiling in the adult Down syndrome brain. Genomics 90:647–660
Robert K, Vialard F, Thiery E, Toyama K, Sinet PM, Janel N, London J (2003) Expression of the cystathionine beta synthase (CBS) gene during mouse development and immunolocalization in adult brain. J Histochem Cytochem 51:363–371
Enokido Y, Suzuki E, Iwasawa K, Namekata K, Okazawa H, Kimura H (2005) Cystathionine β-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J 19:1854–1856
Linden DR, Sha L, Mazzone A, Stoltz GJ, Bernard CE, Furne JK, Levitt MD, Farrugia G et al (2008) Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J Neurochem 106:1577–1585
Lee M, Schwab C, Yu S, McGeer E, McGeer PL (2009) Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol Aging 30:1523–1532
Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR Jr, Darley-Usmar VM et al (2005) Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem 341:40–51
Kabil O, Vitvisky V, Xie P, Banerjee R (2011) The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal 15:363–372
Wang R (2002) Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16:1792–1798
Gadalla MN, Snyder SH (2010) Hydrogen sulfide as a gasotransmitter. J Neurochem 113(1):14–26
Kimura Y, Kimura H (2004) Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18:1165–1167
Kimura H (2000) Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun 267:129–133
Skrajny B, Hannah RS, Roth SH (1992) Low concentrations of hydrogen sulphide alter monoamine levels in the developing rat central nervous system. Can J Physiol Pharmacol 70(11):1515–1518
Talaei F, Bouma HR, Van der Graaf AC, Strijkstra A et al (2011) Serotonin and dopamine protect hypothermia/rewarding damage through the CBS/H2S pathway. PLoS One 6(7):e22568
Butler C, Knox AJ, Bowersox J, Forbes S, Patterson D (2006) The production of transgenic mice expressing human cystathionine beta-synthase to study Down syndrome. Behav Genet 36(3):429–438
Regnier V, Billard JM, Gupta S, Potier B, Woerner S et al (2012) Brain phenotype of transgenic mice overexpressing cystathionine β-synthase. PLoS One 7(1):e29056
Mishima T, Fujiwara T, Kofuji T, Akagawa K (2012) Impairment of catecholamine systems during induction of long-term potentiation at hippocampal CA1 synapses in HPC-1/syntaxin 1A knock-out mice. J Neurosci 32(1):381–389
Chen HB, Wu WN, Wang W, Gu XH, Yu B, Wei B, Yang YJ (2017) Cystathionine-β-synthase-derived hydrogen sulfide is required for amygdalar long-term potentiation and cued fear memory in rats. Pharmacol Biochem Behav 155:16–23. https://doi.org/10.1016/j.pbb.2017.03.002. Epub 2017 Mar 8
Panthi S, Chung HJ, Jung J, Jeong NY (2016) Physiological importance of hydrogen sulfide: emerging potent neuroprotector and neuromodulator. Oxidative Med Cell Longev 2016:9049782. doi: https://doi.org/10.1155/2016/9049782, 1, 11
Moustafa AA, Hewedi DH, Eissa AM, Myers CE, Sadek HA (2012) The relationship between associative learning, transfer generalization, and homocysteine levels in mild cognitive impairment. PLoS One 7(9):e46496. https://doi.org/10.1371/journal.pone.0046496. Epub 2012 Sep 28
Kumar M, Modi M, Sandhir R (2017) Hydrogen sulfide attenuates homocysteine-induced cognitive deficits and neurochemical alterations by improving endogenous hydrogen sulfide levels. Biofactors 43(3):434–450. https://doi.org/10.1002/biof.1354. Epub 2017 Apr 10
Cosgrove KP, Mazure CM, Staley JK (2007) Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62(8):847–855
Jacobson-Pick S, Audet MC, McQuaid RJ, Kalvapalle R, Anisman H (2013) Social agonistic distress in male and female mice: changes of behavior and brain monoamine functioning in relation to acute and chronic challenges. PLoS One8 (4): e 60133, e60133.
Arvidsson E, Viereckel T, Mikulovic S, Wallén-Mackenzie Å (2014) Age-and sex-dependence of dopamine release and capacity for recovery identified in the dorsal striatum of C57/Bl6J mice. PLoS One 9(6):e99592. https://doi.org/10.1371/journal.pone.0099592. eCollection 2014
Velosky AG, Tucker LB, Fu AH, Liu J, McCabe JT (2017) Cognitive performance of male and female C57BL/6J mice after repetitive concussive brain injuries. Behav Brain Res 324:115–124
Block A, Ahmed MM, Dhanasekazran AR, Tong S, Gardiner K (2015) Sex differences in protein expression in the mouse brain and heir perturbations in a model of Down syndrome. Biol Sex Differ 6:24
Ahmed MM, Block A, Tong S, Davisson MT, Gardiner KJ (2017) Age exacerbates abnormal protein expression in a mouse model of Down syndrome. Neurobiol Aging 57:120–132. https://doi.org/10.1016/j.neurobiolaging.2017.05.002
De la Fuente M, Hernanz A, Medina S, Guayerbas N, Fernández B, Viveros MP (2003) Characterization of monoaminergic systems in brain regions of prematurely ageing mice. Neurochem Int 43:165–172
Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP et al (2005) Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacol 49:798–810
Tsunemi A, Utsuyama M, Seidler BK, Kobayashi S, Hirokawa K (2005) Age-related decline of brain monoamines in mice is reversed to young level by Japanese herbal medicine. Neurochem Res 30:75–81
Chourbaji S, Hellweg R, Brandis D, Zörner B, Zacher C, Lang UE, Henn FA, Hörtnagl H et al (2004) Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res Mol Brain Res 121:28–36
Hara Y, Takuma K, Takano E, Katashiba K, Taruta A, Higashino K, Hashimoto H, Ago Y et al (2015) Reduced prefrontal dopaminergic activity in valproic acid-treated mouse autism model. Behav Brain Res 289:39–47. https://doi.org/10.1016/j.bbr.2015.04.022
Akopian G, Crawford C, Beal MF, Cappelletti M, Jakowec MW, Petzinger GM, Zheng L, Gheorghe SL et al J Neurosci 28(38):9585–9597. https://doi.org/10.1523/JNeuroscience.5698-07
Shimohata A, Ishihara K, Hattori S, Miyamoto H, Morishita H, Ornthanalai G, Raveau M, Ebrahim AS et al (2017) Ts1Cje Down syndrome model mice exhibit environmental stimuli-triggered locomotor hyperactivity and sociability concurrent with increased flux through central dopamine and serotonin metabolism. Exp Neurol 293:1–12
Siarey RJ, Stoll J, Rapoport SI, Galdzicki Z (1997) Neuropharmacology altered long-term potentiation in the young and old Ts65Dn mouse, a model for Down Syndrome. Neuropharmacology 36(11–12):1549–1554
Yu T, Liu C, Belichenko P, Clapcote SJ, Li S, Pao A, Kleschevnikov A, Bechard AR et al (2010) Effects of individual segmental trisomies of human chromosome 21 syntenic regions on hippocampal long-term potentiation and cognitive behaviors in mice. Brain Res 1366:162–171. https://doi.org/10.1016/j.brainres.2010.09.107
Ng J, Heales SJ, Kurian MA (2014) Clinical features and pharmacotherapy of childhood monoamine neurotransmitter disorders. Paediatr Drugs 16(4):275–291. https://doi.org/10.1007/s40272-014-0079-z
Winn SR, Scherer T, Thöny B, Harding CO (2016) High dose sapropterin dihydrochloride therapy improves monoamine neurotransmitter turnover in murine phenylketonuria (PKU). Mol Genet Metab 117(1):5–11. https://doi.org/10.1016/j.ymgme.2015.11.012
Vermeiren Y, Janssens J, Aerts T, Martin JJ, Sieben A, Van Dam D, De Deyn PP (2016) Brain serotonergic and noradrenergic deficiencies in behavioral variant frontotemporal dementia compared to early-onset Alzheimer’s disease. J Alzheimer’s Dis 53(3):1079–1096. https://doi.org/10.3233/JAD-160320
Acknowledgements
We thank the personnel of the animal facility of the University Paris-Diderot for taking care of the animals for health and control. We thank for funding CNRS, the European Commission (AnEUploidy project LSHG-CT-2006-037627) and the AFRT (Association Française pour la Recherche sur la Trisomie 21) for grants (2013-2015) and financial support for NFK.
Author information
Authors and Affiliations
Contributions
DJ, LJ, and RC designed the study; BLC, NDK, and RC performed the HPLC experiments; DF, LJ, RC, and SB performed the other experiments; LJ, NDK, and RC analyzed the data; LJ and RC wrote the paper. JN, LS, and MC corrected the manuscript.
Corresponding author
Ethics declarations
This study was approved by the Animal Ethical Committee of University Paris Diderot (CEEA-40, approval number CEB-001-2011).
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(DOCX 20.1 kb)
Rights and permissions
About this article
Cite this article
London, J., Ndiaye, F.K., Bui, L.C. et al. Alterations in the Serotonin and Dopamine Pathways by Cystathionine Beta Synthase Overexpression in Murine Brain. Mol Neurobiol 56, 3958–3971 (2019). https://doi.org/10.1007/s12035-018-1323-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1323-2