Abstract
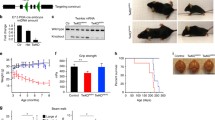
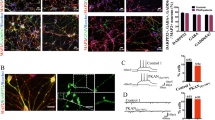
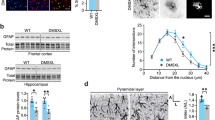
Expression of MeCP2 must be carefully regulated as a reduction or increase results in serious neurological disorders. We are studying transgenic mice in which the MeCP2 gene is expressed at about three times higher than the normal level. Male MeCP2-Tg mice, but not female mice, suffer motor and cognitive deficits and die at 18–20 weeks of age. MeCP2-Tg mice display elevated GFAP and Tau expression within the hippocampus and cortex followed by neuronal loss in these brain regions. Loss of Purkinje neurons, but not of granule neurons in the cerebellar cortex is also seen. Exposure of cultured cortical neurons to either conditioned medium from astrocytes (ACM) derived from male MeCP2-Tg mice or normal astrocytes in which MeCP2 is expressed at elevated levels promotes their death. Interestingly, ACM from male, but not female MeCP2-Tg mice, displays this neurotoxicity reflecting the gender selectivity of neurological symptoms in mice. Male ACM, but not female ACM, contains highly elevated levels of glutamate, and its neurotoxicity can be prevented by MK-801, indicating that it is caused by excitotoxicity. Based on the close phenotypic resemblance of MeCP2-Tg mice to patients with MECP2 triplication syndrome, we suggest for the first time that the human syndrome is a neurodegenerative disorder resulting from astrocyte dysfunction that leads to Tau-mediated excitotoxic neurodegeneration. Loss of cortical and hippocampal neurons may explain the mental retardation and epilepsy in patients, whereas ataxia likely results from the loss of Purkinje neurons.













Similar content being viewed by others
References
Adkins NL, Georgel PT (2011) MeCP2: Structure and function. Biochem Cell Biol (Can) 89:1–11
Guy J, Cheval H, Selfridge J, Bird A (2011) The role of MeCP2 in the brain. Annu Rev Cell Dev Biol (U S) 27:631–652
Hite KC, Adams VH, Hansen JC (2009) Recent advances in MeCP2 structure and function. Biochem Cell Biol (Can) 87:219–227
Ausio J, Martinez de Paz A, Esteller M (2014) MeCP2: The long trip from a chromatin protein to neurological disorders. Trends Mol Med (Engl) 20:487–498
Chahrour M, Zoghbi HY (2007) The story of rett syndrome: From clinic to neurobiology. Neuron (U S) 56:422–437
Lombardi LM, Baker SA, Zoghbi HY (2015) MECP2 disorders: From the clinic to mice and back. J Clin Invest (U S) 125:2914–2923
Peters SU, Hundley RJ, Wilson AK, Carvalho CM, Lupski JR, Ramocki MB (2013) Brief report: Regression timing and associated features in MECP2 duplication syndrome. J Autism Dev Disord 43:2484–2490
Ramocki MB, Tavyev YJ, Peters SU (2010) The MECP2 duplication syndrome. Am J Med Genet A (U S) 152A:1079–1088
Van Esch H (2012) MECP2 duplication syndrome. Mol Syndromol 2:128–136
Tang B, Becanovic K, Desplats PA, Spencer B, Hill AM, Connolly C, Masliah E, Leavitt BR et al (2012a) Forkhead box protein p1 is a transcriptional repressor of immune signaling in the CNS: Implications for transcriptional dysregulation in Huntington disease. Hum Mol Genet (Engl) 21:3097–3111
Lugtenberg D, Kleefstra T, Oudakker AR, Nillesen WM, Yntema HG, Tzschach A, Raynaud M, Rating D et al (2009) Structural variation in Xq28: MECP2 duplications in 1% of patients with unexplained XLMR and in 2% of male patients with severe encephalopathy. Eur J Hum Genet (Engl) 17:444–453
Shimada S, Okamoto N, Ito M, Arai Y, Momosaki K, Togawa M, Maegaki Y, Sugawara M et al (2013) MECP2 duplication syndrome in both genders. Brain Dev (Neth) 35:411–419
Van Esch H, Bauters M, Ignatius J, Jansen M, Raynaud M, Hollanders K, Lugtenberg D, Bienvenu T et al (2005) Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet (U S) 77:442–453
Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, David Sweatt J, Zoghbi HY (2004) Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet (Engl) 13:2679–2689
Jaworski T, Kugler S, Van Leuven F (2010) Modeling of tau-mediated synaptic and neuronal degeneration in alzheimer's disease. Int J Alzheimers Dis (Engl) 2010:1–10. https://doi.org/10.4061/2010/573138
Jaworski T, Dewachter I, Lechat B, Croes S, Termont A, Demedts D, Borghgraef P, Devijver H et al (2009) AAV-tau mediates pyramidal neurodegeneration by cell-cycle re-entry without neurofibrillary tangle formation in wild-type mice. PLoS One (U S) 4:e7280
Min SW et al (2015) Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med (U S) 21:1154–1162
Wang L, Colodner KJ, Feany MB (2011) Protein misfolding and oxidative stress promote glial-mediated neurodegeneration in an alexander disease model. J Neurosci (U S) 31:2868–2877
Bardai FH, Price V, Zaayman M, Wang L, D'Mello SR (2012) Histone deacetylase-1 (HDAC1) is a molecular switch between neuronal survival and death. J Biol Chem (U S) 287:35444–35453
Bardai FH, Verma P, Smith C, Rawat V, Wang L, D'Mello SR (2013) Disassociation of histone deacetylase-3 from normal huntingtin underlies mutant huntingtin neurotoxicity. J Neurosci (U S) 33:11833–11838
Norwood J, Franklin JM, Sharma D, D'Mello SR (2014) Histone deacetylase 3 is necessary for proper brain development. J Biol Chem (U S) 289:34569–34582
Majdzadeh N, Wang L, Morrison BE, Bassel-Duby R, Olson EN, D'Mello SR (2008) HDAC4 inhibits cell-cycle progression and protects neurons from cell death. Dev Neurobiol (U S) 68:1076–1092
Chen HM, Wang L, D'Mello SR (2008) A chemical compound commonly used to inhibit PKR, {8-(imidazol-4-ylmethylene)-6H-azolidino[5,4-g] benzothiazol-7-one}, protects neurons by inhibiting cyclin-dependent kinase. Eur J Neurosci (Fr) 28:2003–2016
Tang SS, Fernandez D, Lazarou LP, Singh R, Fallon P (2012b) MECP2 triplication in 3 brothers - a rarely described cause of familial neurological regression in boys. Eur J Paediatr Neurol (Engl) 16:209–212
Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD (2007) Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci (Engl) 8:427–437
Cerminara NL, Lang EJ, Sillitoe RV, Apps R (2015) Redefining the cerebellar cortex as an assembly of non-uniform purkinje cell microcircuits. Nat Rev Neurosci (Engl) 16:79–93
Maragakis NJ, Rothstein JD (2006) Mechanisms of disease: Astrocytes in neurodegenerative disease. Nat Clin Pract Neurol (Engl) 2:679–689
Bjornsen LP, Eid T, Holmseth S, Danbolt NC, Spencer DD, de Lanerolle NC (2007) Changes in glial glutamate transporters in human epileptogenic hippocampus: Inadequate explanation for high extracellular glutamate during seizures. Neurobiol Dis (U S) 25:319–330
Robel S, Sontheimer H (2016) Glia as drivers of abnormal neuronal activity. Nat Neurosci (U S) 19:28–33
Delépine C, Nectoux J, Bahi-Buisson N, Chelly J, Bienvenu T (2013) MeCP2 deficiency is associated with impaired microtubule stability. (Germany) FEBS Lett 587:245–253
Nectoux J, Florian C, Delepine C, Bahi-Buisson N, Khelfaoui M, Reibel S, Chelly J, Bienvenu T (2012) Altered microtubule dynamics in Mecp2-deficient astrocytes.J Neurosci Res (U S) 90:990–998
Papanikolopoulou K, Skoulakis EM (2011) The power and richness of modelling tauopathies in drosophila. Mol Neurobiol (U S) 44:122–133
Sun XY, Tuo QZ, Liuyang ZY, Xie AJ, Feng XL, Yan X, Qiu M, Li S et al (2016) Extrasynaptic NMDA receptor-induced tau overexpression mediates neuronal death through suppressing survival signaling ERK phosphorylation. Cell Death Dis (Engl) 7:e2449
Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB (2001a) Tauopathy in drosophila: Neurodegeneration without neurofibrillary tangles. Science (U S) 293:711–714
Friez MJ, Jones JR, Clarkson K, Lubs H, Abuelo D, Bier JA, Pai S, Simensen R et al (2006) Recurrent infections, hypotonia, and mental retardation caused by duplication of MECP2 and adjacent region in Xq28. Pediatrics (U S) 118:e1687–e1695
Lubs H, Abidi F, Bier JA, Abuelo D, Ouzts L, Voeller K, Fennell E, Stevenson RE et al (1999) XLMR syndrome characterized by multiple respiratory infections, hypertelorism, severe CNS deterioration and early death localizes to distal Xq28. Am J Med Genet (U S) 85:243–248
Reardon W, Donoghue V, Murphy AM, King MD, Mayne PD, Horn N, Birk Moller L (2010) Progressive cerebellar degenerative changes in the severe mental retardation syndrome caused by duplication of MECP2 and adjacent loci on Xq28. Eur J Pediatr (Germany) 169:941–949
Jiang M, Ash RT, Baker SA, Suter B, Ferguson A, Park J, Rudy J, Torsky SP et al (2013) Dendritic arborization and spine dynamics are abnormal in the mouse model of MECP2 duplication syndrome. J Neurosci (U S) 33:19518–19533
Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, Zoghbi HY (2009) Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum Mol Genet (Engl) 18:2431–2442
Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY (2008) MeCP2, a key contributor to neurological disease, activates and represses transcription. Science (U S) 320:1224–1229
Samaco RC, Mandel-Brehm C, McGraw CM, Shaw CA, McGill BE, Zoghbi HY (2012) Crh and Oprm1 mediate anxiety-related behavior and social approach in a mouse model of MECP2 duplication syndrome. Nat Genet (U S) 44:206–211
Messing A, Brenner M (2003) Alexander disease: GFAP mutations unify young and old. Lancet Neurol (Engl) 2:75
Messing A, Brenner M, Feany MB, Nedergaard M, Goldman JE (2012) Alexander disease. J Neurosci (U S) 32:5017–5023
Yoshida T, Nakagawa M (2012) Clinical aspects and pathology of alexander disease, and morphological and functional alteration of astrocytes induced by GFAP mutation. Neuropathology (Aust) 32:440–446
Hagemann TL, Connor JX, Messing A (2006) Alexander disease-associated glial fibrillary acidic protein mutations in mice induce Rosenthal fiber formation and a white matter stress response. J Neurosci (U S) 26:11162–11173
Jany PL, Hagemann TL, Messing A (2013) GFAP expression as an indicator of disease severity in mouse models of alexander disease. ASN Neuro (Engl) 5:e00109
Tanaka KF, Takebayashi H, Yamazaki Y, Ono K, Naruse M, Iwasato T, Itohara S, Kato H et al (2007) Murine model of alexander disease: Analysis of GFAP aggregate formation and its pathological significance. Glia (U S) 55:617–631
Minkel HR, Anwer TZ, Arps KM, Brenner M, Olsen ML (2015) Elevated GFAP induces astrocyte dysfunction in caudal brain regions: A potential mechanism for hindbrain involved symptoms in type II alexander disease. Glia (U S) 63:2285–2297
Sosunov AA, Guilfoyle E, Wu X, McKhann GM 2nd, Goldman JE (2013) Phenotypic conversions of “protoplasmic” to “reactive” astrocytes in alexander disease. J Neurosci (U S) 33:7439–7450
Tian R, Wu X, Hagemann TL, Sosunov AA, Messing A, McKhann GM, Goldman JE (2010) Alexander disease mutant glial fibrillary acidic protein compromises glutamate transport in astrocytes. J Neuropathol Exp Neurol (U S) 69:335–345
Ilieva H, Polymenidou M, Cleveland DW (2009) Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol (U S) 187:761–772
McGann JC, Lioy DT, Mandel G (2012) Astrocytes conspire with neurons during progression of neurological disease. Curr Opin Neurobiol (Engl) 22:850–858
Sun M, Chen L (2015) Studying tauopathies in drosophila: A fruitful model. Exp Neurol 274:52–57
Boehm J (2013) A ‘danse macabre’: Tau and fyn in STEP with amyloid beta to facilitate induction of synaptic depression and excitotoxicity. Eur J Neurosci (Fr) 37:1925–1930
Couratier P, Lesort M, Terro F, Dussartre C, Hugon J (1996) NMDA antagonist blockade of AT8 tau immunoreactive changes in neuronal cultures. Fundam Clin Pharmacol (Engl) 10:344–349
Esclaire F, Lesort M, Blanchard C, Hugon J (1997) Glutamate toxicity enhances tau gene expression in neuronal cultures. J Neurosci Res (U S) 49:309–318
Sindou P, Couratier P, Barthe D, Hugon J (1992) A dose-dependent increase of tau immunostaining is produced by glutamate toxicity in primary neuronal cultures. Brain Res (Neth) 572:242–246
Pizzi M, Valerio A, Ribola M, Spano PF, Memo M (1993) A tau antisense oligonucleotide decreases neurone sensitivity to excitotoxic injury. Neuroreport (Engl) 4:823–826
Pizzi M, Valerio A, Arrighi V, Galli P, Belloni M, Ribola M, Alberici A, Spano P et al (1995) Inhibition of glutamate-induced neurotoxicity by a tau antisense oligonucleotide in primary culture of rat cerebellar granule cells. Eur J Neurosci (Fr) 7:1603–1613
Cheng JS, Craft R, Yu GQ, Ho K, Wang X, Mohan G, Mangnitsky S, Ponnusamy R et al (2014) Tau reduction diminishes spatial learning and memory deficits after mild repetitive traumatic brain injury in mice. PLoS One (U S) 9:e115765
Ke YD, Suchowerska AK, van der Hoven J, De Silva DM, Wu CW, van Eersel J, Ittner A, Ittner LM (2012) Lessons from tau-deficient mice. Int J Alzheimers Dis (U S) 2012:873270
Morris M, Maeda S, Vossel K, Mucke L (2011) The many faces of tau. Neuron (U S) 70:410–426
Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ et al (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an alzheimer's disease mouse model. Science (U S) 316:750–754
Shipton OA, Leitz JR, Dworzak J, Acton CE, Tunbridge EM, Denk F, Dawson HN, Vitek MP et al (2011) Tau protein is required for amyloid {beta}-induced impairment of hippocampal long-term potentiation. J Neurosci (U S) 31:1688–1692
Acknowledgements
This research was supported by a grant from the National Institutes of Health (R01 NS040408). We thank Dr. Jade Franklin for making significant contributions to the research and the preparation of figures for the manuscript. We also acknowledge the contribution of Dr. Xiaoju Zou for her assistance with some of the immunohistology work described in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplemental Figure 1
(A and B) Locomotor analysis at 15 weeks of age in WT and MeCP2-Tg female mice (n = 6). (C and D) Forelimb and hindlimb grip strength at 15 weeks of age in WT and MeCP2-Tg female mice (n = 6). (E) Brain Weight of 15-week-old WT and MeCP2-Tg female Mice (n = 6). (F) Body Weight of 15-week-old WT and MeCP2-Tg female mice (n = 6). (GIF 28 kb).
Supplemental Figure 2
Male WT and MeCP2-Tg mice (blue), display elevated Tau that correlates to increases in MeCP2 expression (n = 11). Female WT and MeCP2-Tg mice (red) do not display the same correlation (n = 8). (GIF 14 kb).
Rights and permissions
About this article
Cite this article
Montgomery, K.R., Louis Sam Titus, A.S.C., Wang, L. et al. Elevated MeCP2 in Mice Causes Neurodegeneration Involving Tau Dysregulation and Excitotoxicity: Implications for the Understanding and Treatment of MeCP2 Triplication Syndrome. Mol Neurobiol 55, 9057–9074 (2018). https://doi.org/10.1007/s12035-018-1046-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1046-4