Abstract
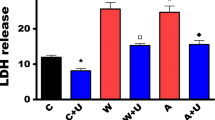
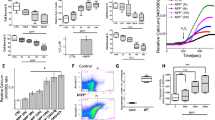
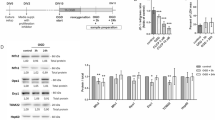
There is emerging evidence suggesting that neurotoxic insults and hypoxic/ischemic injury are underlying causes of Parkinson’s disease (PD). Since PTEN-induced kinase 1 (PINK1) dysfunction is involved in the molecular genesis of PD and since our recent studies have demonstrated that the δ-opioid receptor (DOR) induced neuroprotection against hypoxic and 1-methyl-4-phenyl-pyridimium (MPP+) insults, we sought to explore whether DOR protects neuronal cells from hypoxic and/or MPP+ injury via the regulation of PINK1-related pathways. Using highly differentiated rat PC12 cells exposed to either severe hypoxia (0.5–1% O2) for 24–48 h or varying concentrations of MPP+, we found that both hypoxic and MPP+ stress reduced the level of PINK1 expression, while incubation with the specific DOR agonist UFP-512 reversed this reduction and protected the cells from hypoxia and/or MPP+-induced injury. However, the DOR-mediated cytoprotection largely disappeared after knocking down PINK1 by PINK1 small interfering RNA. Moreover, we examined several important signaling molecules related to cell survival and apoptosis and found that DOR activation attenuated the hypoxic and/or MPP+-induced reduction in phosphorylated Akt and inhibited the activation of cleaved caspase-3, whereas PINK1 knockdown largely deprived the cell of the DOR-induced effects. Our novel data suggests a unique mechanism underlying DOR-mediated cytoprotection against hypoxic and MPP+ stress via a PINK1-mediated regulation of signaling.








Similar content being viewed by others
References
GBD 2013 Mortality and Causes of Death Collaborators (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385(9963):117–171. https://doi.org/10.1016/S0140-6736(14)61682-2
Shulman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6:193–222. https://doi.org/10.1146/annurev-pathol-011110-130242
Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson’s disease. J Neurochem 139(Suppl 1):318–324. https://doi.org/10.1111/jnc.13691
Kazlauskaite A, Muqit MM (2015) PINK1 and Parkin—mitochondrial interplay between phosphorylation and ubiquitylation in Parkinson’s disease. FEBS J 282(2):215–223. https://doi.org/10.1111/febs.13127
Haddad D, Nakamura K (2015) Understanding the susceptibility of dopamine neurons to mitochondrial stressors in Parkinson’s disease. FEBS Lett 589(24 Pt A):3702–3713. https://doi.org/10.1016/j.febslet.2015.10.021
Pickrell AM, Youle RJ (2015) The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85(2):257–273. https://doi.org/10.1016/j.neuron.2014.12.007
Gautier CA, Corti O, Brice A (2014) Mitochondrial dysfunctions in Parkinson’s disease. Rev Neurol 170(5):339–343. https://doi.org/10.1016/j.neurol.2013.06.003
Luo Y, Hoffer A, Hoffer B, Qi X (2015) Mitochondria: a therapeutic target for Parkinson’s disease? Int J Mol Sci 16(9):20704–20730. https://doi.org/10.3390/ijms160920704
Cagin U, Duncan OF, Gatt AP, Dionne MS, Sweeney ST, Bateman JM (2015) Mitochondrial retrograde signaling regulates neuronal function. Proc Natl Acad Sci U S A 112(44):E6000–E6009. https://doi.org/10.1073/pnas.1505036112
Monroy-Jaramillo N, Guerrero-Camacho JL, Rodriguez-Violante M, Boll-Woehrlen MC, Yescas-Gomez P, Alonso-Vilatela ME, Lopez-Lopez M (2014) Genetic mutations in early-onset Parkinson’s disease Mexican patients: molecular testing implications. Am J Med Genet B Neuropsychiatr Genet 165B(3):235–244. https://doi.org/10.1002/ajmg.b.32228
Pridgeon JW, Olzmann JA, Chin LS, Li L (2007) PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol 5(7):e172. https://doi.org/10.1371/journal.pbio.0050172
Wu W, Xu H, Wang Z, Mao Y, Yuan L, Luo W, Cui Z, Cui T et al (2015) PINK1-Parkin-mediated mitophagy protects mitochondrial integrity and prevents metabolic stress-induced endothelial injury. PLoS One 10(7):e0132499. https://doi.org/10.1371/journal.pone.0132499
Dai H, Deng Y, Zhang J, Han H, Zhao M, Li Y, Zhang C, Tian J et al (2015) PINK1/Parkin-mediated mitophagy alleviates chlorpyrifos-induced apoptosis in SH-SY5Y cells. Toxicology 334:72–80. https://doi.org/10.1016/j.tox.2015.06.003
Eiyama A, Okamoto K (2015) PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol 33:95–101. https://doi.org/10.1016/j.ceb.2015.01.002
Kitada T, Tong Y, Gautier CA, Shen J (2009) Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. J Neurochem 111(3):696–702. https://doi.org/10.1111/j.1471-4159.2009.06350.x
Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C et al (2007) Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A 104(27):11441–11446. https://doi.org/10.1073/pnas.0702717104
Ryan BJ, Hoek S, Fon EA, Wade-Martins R (2015) Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci 40(4):200–210. https://doi.org/10.1016/j.tibs.2015.02.003
Chao D, Xia Y (2010) Ionic storm in hypoxic/ischemic stress: can opioid receptors subside it? Prog Neurobiol 90(4):439–470. https://doi.org/10.1016/j.pneurobio.2009.12.007
Sen D, Huchital M, Chen YL (2013) Crosstalk between delta opioid receptor and nerve growth factor signaling modulates neuroprotection and differentiation in rodent cell models. Int J Mol Sci 14(10):21114–21139. https://doi.org/10.3390/ijms141021114
Chen T, Li J, Chao D, Sandhu HK, Liao X, Zhao J, Wen G, Xia Y (2014) Delta-opioid receptor activation reduces alpha-synuclein overexpression and oligomer formation induced by MPP(+) and/or hypoxia. Exp Neurol 255:127–136. https://doi.org/10.1016/j.expneurol.2014.02.022
Xu Y, Zhi F, Shao N, Wang R, Yang Y, Xia Y (2016) Cytoprotection against hypoxic and/or MPP(+) injury: effect of delta-opioid receptor activation on caspase 3. Int J Mol Sci 17(8):1179. https://doi.org/10.3390/ijms17081179
Chao D, Wang Q, Balboni G, Ding G, Xia Y (2016) Attenuating ischemic disruption of K(+) homeostasis in the cortex of hypoxic-ischemic neonatal rats: DOR activation vs. acupuncture treatment. Mol Neurobiol 53(10):7213–7227. https://doi.org/10.1007/s12035-015-9621-4
Borlongan C, Su T, Wang Y (2000) Treatment with delta opioid peptide enhances in vitro and in vivo survival of rat dopaminergic neurons. Neuroreport 11(5):923–926
Borlongan C, Su T, Wang Y (2001) Delta opioid peptide augments functional effects and intrastriatal graft survival of rat fetal ventral mesencephalic cells. Cell Transplant 10(1):53–58
Zhao P, Huang Y, Zuo Z (2006) Opioid preconditioning induces opioid receptor-dependent delayed neuroprotection against ischemia in rats. J Neuropathol Exp Neurol 65(10):945–952
Chiao S, Zuo Z (2014) A double-edged sword: volatile anesthetic effects on the neonatal brain. Brain Sci 4(2):273–294
Chao D, Bazzy-Asaad A, Balboni G, Xia Y (2007) Delta-, but not mu-, opioid receptor stabilizes K(+) homeostasis by reducing Ca(2+) influx in the cortex during acute hypoxia. J Cell Physiol 212(1):60–67. https://doi.org/10.1002/jcp.21000
Ma MC, Qian H, Ghassemi F, Zhao P, Xia Y (2005) Oxygen-sensitive {delta}-opioid receptor-regulated survival and death signals: novel insights into neuronal preconditioning and protection. J Biol Chem 280(16):16208–16218. https://doi.org/10.1074/jbc.M408055200
Zhang J, Gibney GT, Zhao P, Xia Y (2002) Neuroprotective role of delta-opioid receptors in cortical neurons. Am J Physiol Cell Physiol 282(6):C1225–C1234. https://doi.org/10.1152/ajpcell.00226.2001
Saberzadeh J, Arabsolghar R, Takhshid MA (2016) Alpha synuclein protein is involved in aluminum-induced cell death and oxidative stress in PC12 cells. Brain Res 1635:153–160. https://doi.org/10.1016/j.brainres.2016.01.037
Park HJ, Zhao TT, Lee KS, Lee SH, Shin KS, Park KH, Choi HS, Lee MK (2015) Effects of (-)-sesamin on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and dopaminergic neuronal cells of Parkinson’s disease rat models. Neurochem Int 83-84:19–27. https://doi.org/10.1016/j.neuint.2015.01.003
Maioli M, Rinaldi S, Migheli R, Pigliaru G, Rocchitta G, Santaniello S, Basoli V, Castagna A et al (2015) Neurological morphofunctional differentiation induced by REAC technology in PC12. A neuro protective model for Parkinson’s disease. Sci Rep 5:10439. https://doi.org/10.1038/srep10439
Shafer TJ, Atchison WD (1991) Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology 12(3):473–492
Abeliovich A (2014) Neurological disorders: quality-control pathway unlocked. Nature 510(7503):44–45. https://doi.org/10.1038/510044a
Wilhelmus MM, Nijland PG, Drukarch B, de Vries HE, van Horssen J (2012) Involvement and interplay of Parkin, PINK1, and DJ1 in neurodegenerative and neuroinflammatory disorders. Free Radic Biol Med 53(4):983–992. https://doi.org/10.1016/j.freeradbiomed.2012.05.040
Abeliovich A (2010) Parkinson’s disease: mitochondrial damage control. Nature 463(7282):744–745. https://doi.org/10.1038/463744a
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795. https://doi.org/10.1038/nature05292
Wei X, Gao H, Zou J, Liu X, Chen D, Liao J, Xu Y, Ma L et al (2016) Contra-directional coupling of Nur77 and Nurr1 in neurodegeneration: a novel mechanism for memantine-induced anti-inflammation and anti-mitochondrial impairment. Mol Neurobiol 53(9):5876–5892. https://doi.org/10.1007/s12035-015-9477-7
Ordureau A, Heo JM, Duda DM, Paulo JA, Olszewski JL, Yanishevski D, Rinehart J, Schulman BA et al (2015) Defining roles of PARKIN and ubiquitin phosphorylation by PINK1 in mitochondrial quality control using a ubiquitin replacement strategy. Proc Natl Acad Sci U S A 112(21):6637–6642. https://doi.org/10.1073/pnas.1506593112
Ramsay RR, Singer TP (1986) Energy-dependent uptake of N-methyl-4-phenylpyridinium, the neurotoxic metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, by mitochondria. J Biol Chem 261(17):7585–7587
Venderova K, Park DS (2012) Programmed cell death in Parkinson’s disease. Cold Spring Harb Perspect Med 2(8):a009365. https://doi.org/10.1101/cshperspect.a009365
Aksenov V, Long J, Liu J, Szechtman H, Khanna P, Matravadia S, Rollo CD (2013) A complex dietary supplement augments spatial learning, brain mass, and mitochondrial electron transport chain activity in aging mice. Age 35(1):23–33. https://doi.org/10.1007/s11357-011-9325-2
Nagase M, Takahashi Y, Watabe AM, Kubo Y, Kato F (2014) On-site energy supply at synapses through monocarboxylate transporters maintains excitatory synaptic transmission. J Neurosci 34(7):2605–2617. https://doi.org/10.1523/JNEUROSCI.4687-12.2014
Gao J, Zhu ZR, Ding HQ, Qian Z, Zhu L, Ke Y (2007) Vulnerability of neurons with mitochondrial dysfunction to oxidative stress is associated with down-regulation of thioredoxin. Neurochem Int 50(2):379–385. https://doi.org/10.1016/j.neuint.2006.09.007
Devireddy S, Liu A, Lampe T, Hollenbeck PJ (2015) The organization of mitochondrial quality control and life cycle in the nervous system in vivo in the absence of PINK1. J Neurosci 35(25):9391–9401. https://doi.org/10.1523/JNEUROSCI.1198-15.2015
Sanchez-Mora RM, Arboleda H, Arboleda G (2012) PINK1 overexpression protects against C2-ceramide-induced CAD cell death through the PI3K/AKT pathway. J Mol Neurosci 47(3):582–594. https://doi.org/10.1007/s12031-011-9687-z
Dagda RK, Chu CT (2009) Mitochondrial quality control: insights on how Parkinson’s disease related genes PINK1, parkin, and Omi/HtrA2 interact to maintain mitochondrial homeostasis. J Bioenerg Biomembr 41(6):473–479. https://doi.org/10.1007/s10863-009-9255-1
Heeman B, Van den Haute C, Aelvoet SA, Valsecchi F, Rodenburg RJ, Reumers V, Debyser Z, Callewaert G et al (2011) Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J Cell Sci 124(Pt 7):1115–1125. https://doi.org/10.1242/jcs.078303
Koyano F, Matsuda N (2015) Molecular mechanisms underlying PINK1 and Parkin catalyzed ubiquitylation of substrates on damaged mitochondria. Biochim Biophys Acta 1853(10 Pt B):2791–2796. https://doi.org/10.1016/j.bbamcr.2015.02.009
Yang X, Wei A, Liu Y, He G, Zhou Z, Yu Z (2013) IGF-1 protects retinal ganglion cells from hypoxia-induced apoptosis by activating the Erk-1/2 and Akt pathways. Mol Vis 19:1901–1912
Stegeman H, Span PN, Peeters WJ, Verheijen MM, Grenman R, Meijer TW, Kaanders JH, Bussink J (2016) Interaction between hypoxia, AKT and HIF-1 signaling in HNSCC and NSCLC: implications for future treatment strategies. Futur Sci OA 2(1):FSO84. https://doi.org/10.4155/fso.15.84
Zhang Y, Gong XG, Wang ZZ, Sun HM, Guo ZY, Hu JH, Ma L, Li P et al (2016) Overexpression of DJ-1/PARK7, the Parkinson’s disease-related protein, improves mitochondrial function via Akt phosphorylation on threonine 308 in dopaminergic neuron-like cells. Eur J Neurosci 43(10):1379–1388. https://doi.org/10.1111/ejn.13216
Xu Y, Liu C, Chen S, Ye Y, Guo M, Ren Q, Liu L, Zhang H et al (2014) Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cell Signal 26(8):1680–1689. https://doi.org/10.1016/j.cellsig.2014.04.009
Greene LA, Levy O, Malagelada C (2011) Akt as a victim, villain and potential hero in Parkinson’s disease pathophysiology and treatment. Cell Mol Neurobiol 31(7):969–978. https://doi.org/10.1007/s10571-011-9671-8
Murata H, Sakaguchi M, Jin Y, Sakaguchi Y, Futami J, Yamada H, Kataoka K, Huh NH (2011) A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: activation of AKT via mTORC2. J Biol Chem 286(9):7182–7189. https://doi.org/10.1074/jbc.M110.179390
Thornton C, Leaw B, Mallard C, Nair S, Jinnai M, Hagberg H (2017) Cell death in the developing brain after hypoxia-ischemia. Front Cell Neurosci 11:248. https://doi.org/10.3389/fncel.2017.00248
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5(4):a008656. https://doi.org/10.1101/cshperspect.a008656
Liu XR, Li YQ, Hua C, Li SJ, Zhao G, Song HM, Yu MX, Huang Q (2015) Oxidative stress inhibits growth and induces apoptotic cell death in human U251 glioma cells via the caspase-3-dependent pathway. Eur Rev Med Pharmacol Sci 19(21):4068–4075
Meng XW, Fraser MJ, Feller JM, Ziegler JB (2000) Caspase-3-dependent and caspase-3-independent pathways leading to chromatin DNA fragmentation in HL-60 cells. Apoptosis 5(1):61–67
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86(1):147–157
Zhang J, Qian H, Zhao P, Hong SS, Xia Y (2006) Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through delta-opioid receptor. Stroke 37(4):1094–1099. https://doi.org/10.1161/01.STR.0000206444.29930.18
Kim EK, Choi EJ (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 1802(4):396–405. https://doi.org/10.1016/j.bbadis.2009.12.009
Correa SA, Eales KL (2012) The role of p38 MAPK and its substrates in neuronal plasticity and neurodegenerative disease. J Signal Transduction 2012:649079. https://doi.org/10.1155/2012/649079
Funding
This work was supported by the National Natural Science Foundation of China (81590953, 81574053, 81303027), Jiangsu Provincial Special Program of Medical Science (BL2014035), Changzhou Science and Technology Support Program (CE20155060, CE20165048), Changzhou High-Level Medical Talents Training Project (2016CZBJ006), Changzhou Municipal Commissions of Health and Family Planning Major Scientific and Technological Project (ZD201620), Shanghai Key Laboratory of Acupuncture Mechanism and Acupoint Function (14DZ2260500), and Science and Technology Commission of Shanghai Municipality (15441903800).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Dr. Ying Xia is the first corresponding author and Dr. Yilin Yang is the co-corresponding author.
Rights and permissions
About this article
Cite this article
Xu, Y., Zhi, F., Peng, Y. et al. δ-Opioid Receptor Activation Attenuates Hypoxia/MPP+-Induced Downregulation of PINK1: a Novel Mechanism of Neuroprotection Against Parkinsonian Injury. Mol Neurobiol 56, 252–266 (2019). https://doi.org/10.1007/s12035-018-1043-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1043-7