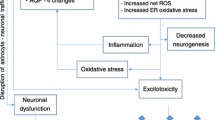
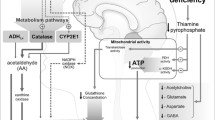
Abstract
Wernicke’s encephalopathy, a common neurological disease, is caused by thiamine (vitamin B1) deficiency. Neuropathy resulting from thiamine deficiency is a hallmark of Wernicke-Korsakoff syndrome in chronic alcohol users. The underlying mechanisms of this deficiency and progression of neuropathy remain to be understood. To uncover the unknown mechanisms of thiamine deficiency in alcohol abuse, we used chronic alcohol consumption or thiamine deficiency diet ingestion in animal models. Observations from animal models were validated in primary human neuronal culture for neurodegenerative process. We employed radio-labeled bio-distribution of thiamine, qualitative and quantitative analyses of the various biomarkers and neurodegenerative process. In the present studies, we established that disruption of thiamine transport across the intestinal gut blood-brain barrier axis as the cause of thiamine deficiency in the brain for neurodegeneration. We found that reduction in thiamine transport across these interfaces was the cause of reduction in the synthesis of thiamine pyrophosphate (TPP), an active cofactor for pyruvate dehydrogenase E1α (PDHE1α). Our findings revealed that decrease in the levels of PDHE1α cofactors switched on the activation of PD kinase (PDK) in the brain, thereby triggering the neuronal phosphorylation of PDHE1α (p-PDHE1α). Dysfunctional phosphorylated PDHE1α causes the reduction of mitochondrial aerobic respiration that led to neurodegeneration. We concluded that impairment of thiamine transport across the gut-BBB-axis that led to insufficient TPP synthesis was critical to Wernicke-neuropathy, which could be effectively prevented by stabilizing the thiamine transporters.










Similar content being viewed by others
References
Kopelman MD (2002) Disorders of memory. Brain: J Neurol 125:2152–2190
Thomson AD, Marshall EJ (2006) The natural history and pathophysiology of Wernicke’s encephalopathy and Korsakoff's psychosis. Alcohol Alcohol (Oxford, Oxfordshire) 41:151–158
Manzo L, Locatelli C, Candura SM, Costa LG (1994) Nutrition and alcohol neurotoxicity. Neurotoxicology 15:555–565
Bakhireva LN, Sharkis J, Shrestha S, Miranda-Sohrabji TJ, Williams S, Miranda RC (2017) Prevalence of prenatal alcohol exposure in the state of Texas as assessed by phosphatidylethanol in newborn dried blood spot specimens. Alcohol Clin Exp Res 41:1004–1011
Bager H, Christensen LP, Husby S, Bjerregaard L (2017) Biomarkers for the detection of prenatal alcohol exposure: a review. Alcohol Clin Exp Res 41:251–261
Fernandes LMP, Bezerra FR, Monteiro MC, Silva ML, de Oliveira FR, Lima RR, Fontes-Junior EA, Maia CSF (2017) Thiamine deficiency, oxidative metabolic pathways and ethanol-induced neurotoxicity: how poor nutrition contributes to the alcoholic syndrome, as Marchiafava-Bignami disease. Eur J Clin Nutr 71:580–586
Boloursaz S, Nekooei S, Seilanian Toosi F, Rezaei-Dalouei H, Davachi B, Kazemi S, Abbasi B (2016) Marchiafava-Bignami and alcohol related acute polyneuropathy: the cooccurrence of two rare entities. Case Rep Neurol Med 2016:5848572
Mehrzad R, Ho MG (2016) Mutism caused by severe demyelination in a patient with Marchiafava-Bignami disease. J Emerg Med 51:e129–e132
Harper CG, Giles M, Finlay-Jones R (1986) Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry 49:341–345
Sechi G, Serra A (2007) Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol 6:442–455
Sparacia G, Anastasi A, Speciale C, Agnello F, Banco A (2017) Magnetic resonance imaging in the assessment of brain involvement in alcoholic and nonalcoholic Wernicke’s encephalopathy. World J Radiol 9:72–78
Nikolakaros G, Ilonen T, Kurki T, Paju J, Papageorgiou SG, Vataja R (2016) Non-alcoholic Korsakoff syndrome in psychiatric patients with a history of undiagnosed Wernicke’s encephalopathy. J Neurol Sci 370:296–302
Segobin S, Ritz L, Lannuzel C, Boudehent C, Vabret F, Eustache F, Beaunieux H, Pitel AL (2015) Integrity of white matter microstructure in alcoholics with and without Korsakoff's syndrome. Hum Brain Mapp 36:2795–2808
Baker KG, Harding AJ, Halliday GM, Kril JJ, Harper CG (1999) Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience 91:429–438
Kopelman MD (2015) What does a comparison of the alcoholic Korsakoff syndrome and thalamic infarction tell us about thalamic amnesia? Neurosci Biobehav Rev 54:46–56
Logan C, Asadi H, Kok HK, Looby ST, Brennan P, O'Hare A, Thornton J (2016) Neuroimaging of chronic alcohol misuse. J Med Imaging Radiat Oncol 61(4):435–440
Sutherland GT, Sheedy D, Kril JJ (2014) Neuropathology of alcoholism. Handb Clin Neurol 125:603–615
Le Berre AP, Pitel AL, Chanraud S, Beaunieux H, Eustache F, Martinot JL, Reynaud M, Martelli C et al (2015) Sensitive biomarkers of alcoholism’s effect on brain macrostructure: similarities and differences between France and the United States. Front Hum Neurosci 9:354
Harper CG, Blumbergs PC (1982) Brain weights in alcoholics. J Neurol Neurosurg Psychiatry 45:838–840
Rommer PS, Fuchs D, Leblhuber F, Schroth R, Greilberger M, Tafeit E, Greilberger J (2016) Lowered levels of carbonyl proteins after vitamin B supplementation in patients with mild cognitive impairment and Alzheimer’s disease. Neurodegener Dis 16:284–289
Pan X, Chen Z, Fei G, Pan S, Bao W, Ren S, Guan Y, Zhong C (2016) Long-term cognitive improvement after benfotiamine administration in patients with Alzheimer’s disease. Neurosci Bull 32:591–596
Zhang Q, Yang G, Li W, Fan Z, Sun A, Luo J, Ke ZJ (2011) Thiamine deficiency increases beta-secretase activity and accumulation of beta-amyloid peptides. Neurobiol Aging 32:42–53
Nolan KA, Black RS, Sheu KF, Langberg J, Blass JP (1991) A trial of thiamine in Alzheimer’s disease. Arch Neurol 48:81–83
Gold M, Chen MF, Johnson K (1995) Plasma and red blood cell thiamine deficiency in patients with dementia of the Alzheimer’s type. Arch Neurol 52:1081–1086
Gibson GE, Hirsch JA, Cirio RT, Jordan BD, Fonzetti P, Elder J (2013) Abnormal thiamine-dependent processes in Alzheimer’s disease. Lessons from diabetes. Mol Cell Neurosci 55:17–25
Butterworth RF, Besnard AM (1990) Thiamine-dependent enzyme changes in temporal cortex of patients with Alzheimer’s disease. Metab Brain Dis 5:179–184
Jimenez-Jimenez FJ, Molina JA, Hernanz A, Fernandez-Vivancos E, de Bustos F, Barcenilla B, Gomez-Escalonilla C, Zurdo M et al (1999) Cerebrospinal fluid levels of thiamine in patients with Parkinson’s disease. Neurosci Lett 271:33–36
Costantini A, Pala MI, Grossi E, Mondonico S, Cardelli LE, Jenner C, Proietti S, Colangeli M et al (2015) Long-term treatment with high-dose thiamine in Parkinson disease: an open-label pilot study. J Altern Complement Med (New York, NY) 21:740–747
Costantini A, Pala MI, Compagnoni L, Colangeli M (2013) High-dose thiamine as initial treatment for Parkinson’s disease. BMJ Case Rep 2013. https://doi.org/10.1136/bcr-2013-009289
Haas RH (1988) Thiamin and the brain. Annu Rev Nutr 8:483–515
Dror V, Rehavi M, Biton IE, Eliash S (2014) Rasagiline prevents neurodegeneration in thiamine deficient rats—a longitudinal MRI study. Brain Res 1557:43–54
Ahmed M, Azizi-Namini P, Yan AT, Keith M (2015) Thiamin deficiency and heart failure: the current knowledge and gaps in literature. Heart Fail Rev 20:1–11
DiNicolantonio JJ, Niazi AK, Lavie CJ, O'Keefe JH, Ventura HO (2013) Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail (Greenwich, Conn) 19:214–222
McCulloch B (2015) High-output heart failure caused by thyrotoxicosis and beriberi. Crit Care Nurs Clin North Am 27:499–510
Koike H, Watanabe H, Inukai A, Iijima M, Mori K, Hattori N, Sobue G (2006) Myopathy in thiamine deficiency: analysis of a case. J Neurol Sci 249:175–179
Hernandez-Vazquez AJ, Garcia-Sanchez JA, Moreno-Arriola E, Salvador-Adriano A, Ortega-Cuellar D, Velazquez-Arellano A (2016) Thiamine deprivation produces a liver ATP deficit and metabolic and genomic effects in mice: findings are parallel to those of biotin deficiency and have implications for energy disorders. J Nutrigenet Nutrigenomics 9:287–299
Zahr NM, Alt C, Mayer D, Rohlfing T, Manning-Bog A, Luong R, Sullivan EV, Pfefferbaum A (2014) Associations between in vivo neuroimaging and postmortem brain cytokine markers in a rodent model of Wernicke's encephalopathy. Exp Neurol 261:109–119
Liu D, Ke Z, Luo J (2017) Thiamine deficiency and Neurodegeneration: the interplay among oxidative stress, endoplasmic reticulum stress, and autophagy. Mol Neurobiol 54(7):5440–5448
Wang X, Xu M, Frank JA, Ke ZJ, Luo J (2017) Thiamine deficiency induces endoplasmic reticulum stress and oxidative stress in human neurons derived from induced pluripotent stem cells. Toxicol Appl Pharmacol 320:26–31
Hazell AS, Faim S, Wertheimer G, Silva VR, Marques CS (2013) The impact of oxidative stress in thiamine deficiency: a multifactorial targeting issue. Neurochem Int 62(5):796–802
Abdou E, Hazell AS (2015) Thiamine deficiency: an update of pathophysiologic mechanisms and future therapeutic considerations. Neurochem Res 40:353–361
Reidling JC, Lambrecht N, Kassir M, Said HM (2010) Impaired intestinal vitamin B1 (thiamin) uptake in thiamin transporter-2-deficient mice. Gastroenterology 138:1802–1809
Hoyumpa AM Jr (1980) Mechanisms of thiamin deficiency in chronic alcoholism. Am J Clin Nutr 33:2750–2761
Latt N, Dore G (2014) Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J 44:911–915
Subramanya SB, Subramanian VS, Said HM (2010) Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol 299:G23–G31
Abdul Muneer PM, Alikunju S, Szlachetka AM, Murrin LC, Haorah J (2011) Impairment of brain endothelial glucose transporter by methamphetamine causes blood-brain barrier dysfunction. Mol Neurodegener 6:23
Haorah J, Floreani NA, Knipe B, Persidsky Y (2011) Stabilization of superoxide dismutase by acetyl-l-carnitine in human brain endothelium during alcohol exposure: novel protective approach. Free Radic Biol Med 51:1601–1609
Haorah J, Rump TJ, Xiong H (2013) Reduction of brain mitochondrial beta-oxidation impairs complex I and V in chronic alcohol intake: the underlying mechanism for neurodegeneration. PLoS One 8:e70833
Butterworth RF, Kril JJ, Harper CG (1993) Thiamine-dependent enzyme changes in the brains of alcoholics: relationship to the Wernicke-Korsakoff syndrome. Alcohol Clin Exp Res 17:1084–1088
Qin L, Crews FT (2014) Focal thalamic degeneration from ethanol and thiamine deficiency is associated with neuroimmune gene induction, microglial activation, and lack of monocarboxylic acid transporters. Alcohol Clin Exp Res 38:657–671
Wijnia JW, Oudman E (2013) Biomarkers of delirium as a clue to diagnosis and pathogenesis of Wernicke-Korsakoff syndrome. Eur J Neurol 20:1531–1538
Acknowledgements
The National Institute of Health (NIH/NIAAA) supported the work.
Funding
This work was supported by NIH/NIAAA grant 1R21AA022734-01A1, R21 AA020370-01A1 (to JH).
Author information
Authors and Affiliations
Contributions
PMAM carried out the studies, performed the acquisition data and involved in manuscript preparation. HS and SA assisted PMAM in experiments and data acquisition. AMS performed the animal care, and pair-feeding, XM helped JH with the manuscript preparation and figure design. JH designed the whole project, supervised the experiments, data interpretation and prepared the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
Elective abortus specimens of human fetal brain tissues were obtained in full compliance with the ethical guidelines of the National Institutes of Health and University of Nebraska Medical Center. No disclosure of the source of abortus tissue or of patient information was possible since only de-identified abortus tissues were obtained from the source. In all instances, informed consent was obtained and maintained by the source.
Consent for Publication
Not applicable.
Availability of Data and Materials
All available are presented in this main manuscript.
Competing Interests
The authors declare that they have competing interests.
Rights and permissions
About this article
Cite this article
Abdul-Muneer, P.M., Alikunju, S., Schuetz, H. et al. Impairment of Thiamine Transport at the GUT-BBB-AXIS Contributes to Wernicke’s Encephalopathy. Mol Neurobiol 55, 5937–5950 (2018). https://doi.org/10.1007/s12035-017-0811-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0811-0