Abstract
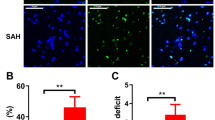
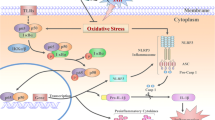
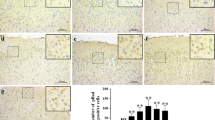
Subarachnoid hemorrhage (SAH), as a severe brain disease, has high morbidity and mortality. SAH usually induced neurological dysfunction or death and the treatment is far from satisfaction. Here, we investigated the effect of low dose of LPS pretreatment and underlying molecular mechanism in rat SAH model. Firstly, SAH model was induced by prechiasmal cistern injection method (SAH1) and common carotid artery-prechiasmal cistern shunt method (SAH2), respectively, to select the more suitable SAH model. At 6, 12, 24, 48, and 72 h after SAH, brain injury including neurological dysfunction, blood–brain barrier disruption, brain edema, and cell apoptosis were detected. And the expression of MMP9, HMGB1/TLR4, and caspase3 in cortex were also explored. Then, SB-3CT, an inhibitor of MMP9, was administrated to investigate the exact function of MMP9 in the brain injury at 24 h after SAH. Moreover, low dose of LPS was used to verify whether it had nerve protection after SAH and the mechanism involving in MMP9 and caspase 3 was investigated. Our results showed SAH1 seems to be the most suitable SAH model. In addition, MMP9 activated by HMGB1/TLR4 may promote or aggravate brain injury, while inhibiting MMP9 via SB-3CT exerted a neuroprotective effect. Moreover, LPS improved the neurological dysfunction, reduced Evans blue extravasation and brain edema, and inhibited cell apoptosis of cortex in rats with brain injury induced by SAH. Importantly, LPS pretreatment increased the expression level of TLR4, and decreased the level of MMP9 and caspase3. Therefore, the present study revealed that low dose of LPS pretreatment could provide neuroprotective effects on brain injury caused by SAH via downregulating MMP9 and caspase3 and activating TLR4 signal pathway.








Similar content being viewed by others
Change history
06 December 2016
An erratum to this article has been published.
Reference
Broderick JP, Brott TG, Duldner JE et al (1994) Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 25(7):1342–1347
Edner G, Kagstrom E, Wallstedt L (1992) Total overall management and surgical outcome after aneurysmal subarachnoid haemorrhage in a defined population. Br J Neurosurg 6(5):409–420
Hutter BO, Kreitschmann-Andermahr I, Gilsbach JM (2001) Health-related quality of life after aneurysmal subarachnoid hemorrhage: impacts of bleeding severity, computerized tomography findings, surgery, vasospasm, and neurological grade. J Neurosurg 94(2):241–251
Ljunggren B, Saveland H, Brandt L et al (1984) Aneurysmal subarachnoid hemorrhage. Total annual outcome in a 1.46 million population. Surg Neurol 22(5):435–438
Crompton MR (1964) The pathogenesis of cerebral infarction following the rupture of cerebral berry aneurysms. Brain 87:491–510
Bederson JB, Germano IM, Guarino L (1995) Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke 26(6):1086–1091 discussion 1091-2
Bederson JB, Levy AL, Ding WH et al (1998) Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery 42(2):352–360 discussion 360-2
Jackowski A, Crockard A, Burnstock G et al (1990) The time course of intracranial pathophysiological changes following experimental subarachnoid haemorrhage in the rat. J Cereb Blood Flow Metab 10(6):835–849
Prunell GF, Mathiesen T, Diemer NH et al (2003) Experimental subarachnoid hemorrhage: subarachnoid blood volume, mortality rate, neuronal death, cerebral blood flow, and perfusion pressure in three different rat models. Neurosurgery 52(1):165–175 discussion 175-6
Schwartz AY, Masago A, Sehba FA et al (2000) Experimental models of subarachnoid hemorrhage in the rat: a refinement of the endovascular filament model. J Neurosci Methods 96(2):161–167
Veelken JA, Laing RJ, Jakubowski J (1995) The Sheffield model of subarachnoid hemorrhage in rats. Stroke 26(7):1279–1283 discussion 1284
Zhao W, Ujiie H, Tamano Y et al (1999) Sudden death in a rat subarachnoid hemorrhage model. Neurol Med Chir (Tokyo) 39(11):735–741 discussion 741-3
Cahill J, Calvert JW, Zhang JH (2006) Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 26(11):1341–1353
Kusaka G, Ishikawa M, Nanda A et al (2004) Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 24(8):916–925
Bazan NG, Rodriguez de Turco EB (1980) Membrane lipids in the pathogenesis of brain edema: phospholipids and arachidonic acid, the earliest membrane components changed at the onset of ischemia. Adv Neurol 28:197–205
Park S, Yamaguchi M, Zhou C et al (2004) Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke 35(10):2412–2417
Hamann GF, Okada Y, Fitridge R et al (1995) Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke 26(11):2120–2126
Gu Z, Cui J, Brown S et al (2005) A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci 25(27):6401–6408
Guo Z, Sun X, He Z et al (2010) Matrix metalloproteinase-9 potentiates early brain injury after subarachnoid hemorrhage. Neurol Res 32(7):715–720
Guo ZD, Zhang XD, Wu HT et al (2011) Matrix metalloproteinase 9 inhibition reduces early brain injury in cortex after subarachnoid hemorrhage. Acta Neurochir Suppl. 110(Pt 1):81–84
Qiu J, Xu J, Zheng Y et al (2010) High-mobility group box 1 promotes metalloproteinase-9 upregulation through toll-like receptor 4 after cerebral ischemia. Stroke 41(9):2077–2082
Rosenzweig HL, Lessov NS, Henshall DC et al (2004) Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke 35(11):2576–2581
Rosenzweig HL, Minami M, Lessov NS et al (2007) Endotoxin preconditioning protects against the cytotoxic effects of TNFalpha after stroke: a novel role for TNFalpha in LPS-ischemic tolerance. J Cereb Blood Flow Metab 27(10):1663–1674
Hang CH, Shi JX, Tian J, Li JS, Wu W, Yin HX (2004) Effect of systemic LPS injection on cortical NF-kappaB activity and inflammatory response following traumatic brain injury in rats. Brain Res 1026(1):23–32
Pradillo JM, Fernandez-Lopez D, Garcia-Yebenes I et al (2009) Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J Neurochem 109(1):287–294
Hu Q, Chen C, Yan J, Yang X, Shi X, Zhao J, Lei J, Yang L et al (2009) Therapeutic application of gene silencing MMP-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp Neurol 216:35–46
Lenzsér G, Kis B, Snipes JA, Gáspár T, Sándor P, Komjáti K, Szabó C, Busija DW (2007) Contribution of poly (ADP-ribose) polymerase to postischemic blood–brain barrier damage in rats. J Cereb Blood Flow Metab 27:1318–1326
Masuda T, Sato K, Yamamoto S et al (2002) Sympathetic nervous activity and myocardial damage immediately after subarachnoid hemorrhage in a unique animal model. Stroke 33(6):1671–1676
Pradilla G, Thai QA, Legnani FG et al (2005) Local delivery of ibuprofen via controlled-release polymers prevents angiographic vasospasm in a monkey model of subarachnoid hemorrhage. Neurosurgery 57(1 Suppl):184–190 discussion 184-90
Westermaier T, Jauss A, Eriskat J et al (2009) Time-course of cerebral perfusion and tissue oxygenation in the first 6 h after experimental subarachnoid hemorrhage in rats. J Cereb Blood Flow Metab 29(4):771–779
Yin YH, Wang F, Pan YH et al (2009) Effects of dose-response of topical administration of nimodipine on cerebral vasospasm after subarachnoid hemorrhage in rabbits. Am J Med Sci 337(2):123–125
Lindegaard KF (1999) The role of transcranial Doppler in the management of patients with subarachnoid haemorrhage—a review. Acta Neurochir Suppl 72:59–71
Delgado TJ, Brismar J, Svendgaard NA (1985) Subarachnoid haemorrhage in the rat: angiography and fluorescence microscopy of the major cerebral arteries. Stroke 16(4):595–602
Solomon RA, Antunes JL, Chen RY, Bland L, Chien S (1985) Decrease in cerebral blood flow in rats after experimental subarachnoid hemorrhage: a new animal model. Stroke 16(1):58–64
Delgado TJ, Diemer NH, Svendgaard NA (1986) Subarachnoid hemorrhage in the rat: cerebral blood flow and glucose metabolism after selective lesions of the catecholamine systems in the brainstem. J Cereb Blood Flow Metab 6(5):600–606
Ram Z, Sahar A, Hadani M (1991) Vasospasm due to massive subarachnoid haemorrhage—a rat model. Acta Neurochir 110(3–4):181–184
Vikman P, Beg S, Khurana TS et al (2006) Gene expression and molecular changes in cerebral arteries following subarachnoid hemorrhage in the rat. J Neurosurg 105(3):438–444
Castillo J, Leira R, Blanco M (2004) Metalloproteinases and neurovascular injury. Neurologia 19(6):312–320
Zhao BQ, Tejima E, Lo EH (2007) Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke 38(2 Suppl):748–752
Kiaei M, Kipiani K, Calingasan NY et al (2007) Matrix metalloproteinase-9 regulates TNF-alpha and FasL expression in neuronal, glial cells and its absence extends life in a transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol 205(1):74–81
Fukuda S, Fini CA, Mabuchi T et al (2004) Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke 35(4):998–1004
Zalewska T, Ziemka-Nalecz M, Sarnowska A et al (2003) Transient forebrain ischemia modulates signal transduction from extracellular matrix in gerbil hippocampus. Brain Res 977(1):62–69
Rosell A, Cuadrado E, Ortega-Aznar A et al (2008) MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 39(4):1121–1126
Yu M, Wang H, Ding A et al (2006) HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26(2):174–179
Park JS, Svetkauskaite D, He Q et al (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279(9):7370–7377
van Zoelen MA, Yang H, Florquin S et al (2009) Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock 31(3):280–284
Kruger B, Krick S, Dhillon N et al (2009) Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A 106(9):3390–3395
Wang H, Bloom O, Zhang M et al (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285(5425):248–251
Tasaki K, Ruetzler CA, Ohtsuki T et al (1997) Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res 748(1–2):267–270
Acknowledgements
This research was supported by a grant of the National Science Foundation of China (No. 81271326, No.81100910), and supported by the Program for IRTSTYN, together with program Innovative Research Team In Science and Technology in Yunnan province (2016–2019).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Ting-Hua Wang, Liu-Lin Xiong, Shuai-Fen Yang, Shu-Fen Wang and, Jia-Liu contributed equally to this work.
The original version of this article was revised: The author's name Shu-Feng Wang was changed to Shu-Fen Wang per request of authors.
An erratum to this article is available at https://doi.org/10.1007/s12035-016-0320-6.
Rights and permissions
About this article
Cite this article
Wang, TH., Xiong, LL., Yang, SF. et al. LPS Pretreatment Provides Neuroprotective Roles in Rats with Subarachnoid Hemorrhage by Downregulating MMP9 and Caspase3 Associated with TLR4 Signaling Activation. Mol Neurobiol 54, 7746–7760 (2017). https://doi.org/10.1007/s12035-016-0259-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0259-7