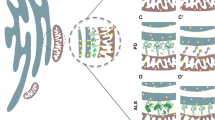
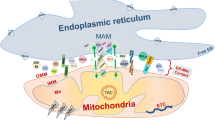
Abstract
Mitochondria-associated membranes (MAMs) are structures that regulate physiological functions between endoplasmic reticulum (ER) and mitochondria in order to maintain calcium signaling and mitochondrial biogenesis. Several proteins located in MAMs, including those encoded by PARK genes and some of neurodegeneration-related proteins (huntingtin, presenilin, etc.), ensure this regulation. In this regard, MAM alteration is associated with neurodegenerative diseases such as Parkinson’s (PD), Alzheimer’s (AD), and Huntington’s diseases (HD) and contributes to the appearance of the pathogenesis features, i.e., autophagy dysregulation, mitochondrial dysfunction, oxidative stress, and lately, neuronal death. Moreover,, ER stress and/or damaged mitochondria can be the cause of these disruptions. Therefore, ER-mitochondria contact structure and function are crucial to multiple cellular processes. This review is focused on the molecular interaction between ER and mitochondria indispensable to MAM formation and on MAM alteration-induced etiology of neurodegenerative diseases.

Similar content being viewed by others
References
Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK (2011) ER tubules mark sites of mitochondrial division. Science 334(6054):358–362. doi:10.1126/science.1207385
Shim SH, Xia C, Zhong G, Babcock HP, Vaughan JC, Huang B, Wang X, Xu C, Bi GQ, Zhuang X (2012) Super-resolution fluorescence imaging of organelles in live cells with photoswitchable membrane probes. Proc Natl Acad Sci U S A 109(35):13978–13983. doi:10.1073/pnas.1201882109
Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A, Agostinis P (2012) PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 19(11):1880–1891. doi:10.1038/cdd.2012.74
Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, Amano A, Yoshimori T (2013) Autophagosomes form at ER-mitochondria contact sites. Nature 495(7441):389–393. doi:10.1038/nature11910
Szabadkai G, Rizzuto R (2013) Kalphalambdaovarsigma kappaalphaiota Agammaalphathetaovarsigma: how mitochondrial beauty translates into biological virtue. Curr Opin Cell Biol 25(4):477–482. doi:10.1016/j.ceb.2013.03.006
Area-Gomez E, Del Carmen Lara Castillo M, MD T, Guardia-Laguarta C, AJ d G, Madra M, Ikenouchi J, Umeda M, TD B, SL S, EA S (2012) Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J 31(21):4106–4123. doi:10.1038/emboj.2012.202
Hedskog L, Pinho CM, Filadi R, Ronnback A, Hertwig L, Wiehager B, Larssen P, Gellhaar S, Sandebring A, Westerlund M, Graff C, Winblad B, Galter D, Behbahani H, Pizzo P, Glaser E, Ankarcrona M (2013) Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc Natl Acad Sci U S A 110(19):7916–7921. doi:10.1073/pnas.1300677110
Sheftel AD, Zhang AS, Brown C, Shirihai OS, Ponka P (2007) Direct interorganellar transfer of iron from endosome to mitochondrion. Blood 110(1):125–132. doi:10.1182/blood-2007-01-068148
Daniele T, Hurbain I, Vago R, Casari G, Raposo G, Tacchetti C, Schiaffino MV (2014) Mitochondria and melanosomes establish physical contacts modulated by Mfn2 and involved in organelle biogenesis. Current biology : CB 24(4):393–403. doi:10.1016/j.cub.2014.01.007
Ungermann C (2015) vCLAMPs-an intimate link between vacuoles and mitochondria. Curr Opin Cell Biol 35:30–36. doi:10.1016/j.ceb.2015.03.006
Islinger M, Luers GH, Zischka H, Ueffing M, Volkl A (2006) Insights into the membrane proteome of rat liver peroxisomes: microsomal glutathione-S-transferase is shared by both subcellular compartments. Proteomics 6(3):804–816. doi:10.1002/pmic.200401347
Jourdain I, Sontam D, Johnson C, Dillies C, Hyams JS (2008) Dynamin-dependent biogenesis, cell cycle regulation and mitochondrial association of peroxisomes in fission yeast. Traffic 9(3):353–365. doi:10.1111/j.1600-0854.2007.00685.x
Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, McBride HM (2008) Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Current biology : CB 18(2):102–108. doi:10.1016/j.cub.2007.12.038
Elbaz-Alon Y, Rosenfeld-Gur E, Shinder V, Futerman AH, Geiger T, Schuldiner M (2014) A dynamic interface between vacuoles and mitochondria in yeast. Dev Cell 30(1):95–102. doi:10.1016/j.devcel.2014.06.007
Honscher C, Mari M, Auffarth K, Bohnert M, Griffith J, Geerts W, van der Laan M, Cabrera M, Reggiori F, Ungermann C (2014) Cellular metabolism regulates contact sites between vacuoles and mitochondria. Dev Cell 30(1):86–94. doi:10.1016/j.devcel.2014.06.006
Copeland DE, Dalton AJ (1959) An association between mitochondria and the endoplasmic reticulum in cells of the pseudobranch gland of a teleost. J Biophys Biochem Cytol 5(3):393–396
Pickett CB, Montisano D, Eisner D, Cascarano J (1980) The physical association between rat liver mitochondria and rough endoplasmic reticulum. I. Isolation, electron microscopic examination and sedimentation equilibrium centrifugation analyses of rough endoplasmic reticulum-mitochondrial complexes. Exp Cell Res 128(2):343–352
Montisano DF, Cascarano J, Pickett CB, James TW (1982) Association between mitochondria and rough endoplasmic reticulum in rat liver. Anat Rec 203(4):441–450. doi:10.1002/ar.1092030403
Vance JE (1990) Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 265(13):7248–7256
McMurray WC, Dawson RM (1969) Phospholipid exchange reactions within the liver cell. The Biochemical Journal 112(1):91–108
Jungalwala FB, Dawson RM (1970) The origin of mitochondrial phosphatidylcholine within the liver cell. European Journal of Biochemistry/FEBS 12(2):399–402
Sauner MT, Levy M (1971) Study of the transfer of phospholipids from the endoplasmic reticulum to the outer and inner mitochondrial membranes. J Lipid Res 12(1):71–75
Dennis EA, Kennedy EP (1972) Intracellular sites of lipid synthesis and the biogenesis of mitochondria. J Lipid Res 13(2):263–267
Stone SJ, Vance JE (2000) Phosphatidylserine synthase-1 and −2 are localized to mitochondria-associated membranes. J Biol Chem 275(44):34534–34540. doi:10.1074/jbc.M002865200
Cui Z, Vance JE, Chen MH, Voelker DR, Vance DE (1993) Cloning and expression of a novel phosphatidylethanolamine N-methyltransferase. A specific biochemical and cytological marker for a unique membrane fraction in rat liver. J Biol Chem 268(22):16655–16663
Rusinol AE, Cui Z, Chen MH, Vance JE (1994) A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J Biol Chem 269(44):27494–27502
Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280(5370):1763–1766
Szabadkai G, Simoni AM, Rizzuto R (2003) Mitochondrial Ca2+ uptake requires sustained Ca2+ release from the endoplasmic reticulum. J Biol Chem 278(17):15153–15161. doi:10.1074/jbc.M300180200
Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R (2006) Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175(6):901–911. doi:10.1083/jcb.200608073
Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH (2003) Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol 5(12):1051–1061. doi:10.1038/ncb1063
Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK (2010) Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142(2):270–283. doi:10.1016/j.cell.2010.06.007
Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, Rodrigues MA, Gomez MV, Nathanson MH, Leite MF (2005) The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem 280(49):40892–40900. doi:10.1074/jbc.M506623200
Rizzuto R, Pozzan T (2006) Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev 86(1):369–408. doi:10.1152/physrev.00004.2005
Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G (2006) Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174(7):915–921. doi:10.1083/jcb.200604016
Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P (2009) An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325(5939):477–481. doi:10.1126/science.1175088
Murley A, Lackner LL, Osman C, West M, Voeltz GK, Walter P, Nunnari J (2013) ER-associated mitochondrial division links the distribution of mitochondria and mitochondrial DNA in yeast. eLife 2:e00422. doi:10.7554/eLife.00422
Wang PT, Garcin PO, Fu M, Masoudi M, St-Pierre P, Pante N, Nabi IR (2015) Distinct mechanisms controlling rough and smooth endoplasmic reticulum contacts with mitochondria. J Cell Sci 128(15):2759–2765. doi:10.1242/jcs.171132
Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G (2005) PACS-2 controls endoplasmic reticulum-mitochondria communication and bid-mediated apoptosis. EMBO J 24(4):717–729. doi:10.1038/sj.emboj.7600559
de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456(7222):605–610. doi:10.1038/nature07534
Myhill N, Lynes EM, Nanji JA, Blagoveshchenskaya AD, Fei H, Carmine Simmen K, Cooper TJ, Thomas G, Simmen T (2008) The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell 19(7):2777–2788. doi:10.1091/mbc.E07-10-0995
Kottgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Hopker K, Simmen KC, Tschucke CC, Sandford R, Kim E, Thomas G, Walz G (2005) Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J 24(4):705–716. doi:10.1038/sj.emboj.7600566
Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P (2015) Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc Natl Acad Sci U S A 112(17):E2174–E2181. doi:10.1073/pnas.1504880112
Cosson P, Marchetti A, Ravazzola M, Orci L (2012) Mitofusin-2 independent juxtaposition of endoplasmic reticulum and mitochondria: an ultrastructural study. PLoS One 7(9):e46293. doi:10.1371/journal.pone.0046293
De Vos KJ, Morotz GM, Stoica R, Tudor EL, Lau KF, Ackerley S, Warley A, Shaw CE, Miller CC (2012) VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum Mol Genet 21(6):1299–1311. doi:10.1093/hmg/ddr559
Stoica R, De Vos KJ, Paillusson S, Mueller S, Sancho RM, Lau KF, Vizcay-Barrena G, Lin WL, Xu YF, Lewis J, Dickson DW, Petrucelli L, Mitchell JC, Shaw CE, Miller CC (2014) ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat Commun 5:3996. doi:10.1038/ncomms4996
Huttlin EL, Ting L, Bruckner RJ, Gebreab F, Gygi MP, Szpyt J, Tam S, Zarraga G, Colby G, Baltier K, Dong R, Guarani V, Vaites LP, Ordureau A, Rad R, Erickson BK, Wuhr M, Chick J, Zhai B, Kolippakkam D, Mintseris J, Obar RA, Harris T, Artavanis-Tsakonas S, Sowa ME, De Camilli P, Paulo JA, Harper JW, Gygi SP (2015) The BioPlex network: a systematic exploration of the human Interactome. Cell 162(2):425–440. doi:10.1016/j.cell.2015.06.043
Mizuno K, Padma P, Konno A, Satouh Y, Ogawa K, Inaba K (2009) A novel neuronal calcium sensor family protein, calaxin, is a potential Ca(2+)-dependent regulator for the outer arm dynein of metazoan cilia and flagella. Biology of the Cell/Under the Auspices of the European Cell Biology Organization 101(2):91–103. doi:10.1042/BC20080032
Vinogradova MV, Malanina GG, Reddy AS, Fletterick RJ (2009) Structure of the complex of a mitotic kinesin with its calcium binding regulator. Proc Natl Acad Sci U S A 106(20):8175–8179. doi:10.1073/pnas.0811131106
Yi M, Weaver D, Hajnoczky G (2004) Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol 167(4):661–672. doi:10.1083/jcb.200406038
Pizzo P, Pozzan T (2007) Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol 17(10):511–517. doi:10.1016/j.tcb.2007.07.011
Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnoczky G (2008) Bidirectional Ca2 + −dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A 105(52):20728–20733. doi:10.1073/pnas.0808953105
Fransson S, Ruusala A, Aspenstrom P (2006) The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun 344(2):500–510. doi:10.1016/j.bbrc.2006.03.163
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160(2):189–200. doi:10.1083/jcb.200211046
Youle RJ, van der Bliek AM (2012) Mitochondrial fission, fusion, and stress. Science 337(6098):1062–1065. doi:10.1126/science.1219855
Hoppins S, Nunnari J (2012) Cell biology. Mitochondrial dynamics and apoptosis—the ER connection. Science 337(6098):1052–1054. doi:10.1126/science.1224709
Loson OC, Song Z, Chen H, Chan DC (2013) Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell 24(5):659–667. doi:10.1091/mbc.E12-10-0721
Arasaki K, Shimizu H, Mogari H, Nishida N, Hirota N, Furuno A, Kudo Y, Baba M, Baba N, Cheng J, Fujimoto T, Ishihara N, Ortiz-Sandoval C, Barlow LD, Raturi A, Dohmae N, Wakana Y, Inoue H, Tani K, Dacks JB, Simmen T, Tagaya M (2015) A role for the ancient SNARE syntaxin 17 in regulating mitochondrial division. Dev Cell 32(3):304–317. doi:10.1016/j.devcel.2014.12.011
Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al. (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8(4):445–544
Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT (2008) Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182(4):685–701. doi:10.1083/jcb.200803137
Ge L, Melville D, Zhang M, Schekman R (2013) The ER-Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis. eLife 2:e00947. doi:10.7554/eLife.00947
Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J (2010) Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 141(4):656–667. doi:10.1016/j.cell.2010.04.009
Ravikumar B, Moreau K, Rubinsztein DC (2010) Plasma membrane helps autophagosomes grow. Autophagy 6(8):1184–1186. doi:10.4161/auto.6.8.13428
Bockler S, Westermann B (2014) ER-mitochondria contacts as sites of mitophagosome formation. Autophagy 10(7):1346–1347. doi:10.4161/auto.28981
Rodriguez-Enriquez S, Kim I, Currin RT, Lemasters JJ (2006) Tracker dyes to probe mitochondrial autophagy (mitophagy) in rat hepatocytes. Autophagy 2(1):39–46
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124(3):471–484. doi:10.1016/j.cell.2006.01.016
Betz C, Stracka D, Prescianotto-Baschong C, Frieden M, Demaurex N, Hall MN (2013) Feature article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Natl Acad Sci U S A 110(31):12526–12534. doi:10.1073/pnas.1302455110
Somlyo AP (1984) Cell physiology: cellular site of calcium regulation. Nature 309(5968):516–517
Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, Pozzan T (2010) Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell 38(2):280–290. doi:10.1016/j.molcel.2010.04.003
Michalak M, Robert Parker JM, Opas M (2002) Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium 32(5–6):269–278
Joseph SK, Boehning D, Bokkala S, Watkins R, Widjaja J (1999) Biosynthesis of inositol trisphosphate receptors: selective association with the molecular chaperone calnexin. The Biochemical Journal 342(Pt 1):153–161
John LM, Lechleiter JD, Camacho P (1998) Differential modulation of SERCA2 isoforms by calreticulin. J Cell Biol 142(4):963–973
Lynes EM, Raturi A, Shenkman M, Ortiz Sandoval C, Yap MC, Wu J, Janowicz A, Myhill N, Benson MD, Campbell RE, Berthiaume LG, Lederkremer GZ, Simmen T (2013) Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling. J Cell Sci 126(Pt 17):3893–3903. doi:10.1242/jcs.125856jcs.125856
Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131(3):596–610. doi:10.1016/j.cell.2007.08.036
Keinan N, Pahima H, Ben-Hail D, Shoshan-Barmatz V (2013) The role of calcium in VDAC1 oligomerization and mitochondria-mediated apoptosis. Biochim Biophys Acta 1833(7):1745–1754. doi:10.1016/j.bbamcr.2013.03.017
Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, Tuft RA, Fogarty KE, Rizzuto R (2002) Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol 159(4):613–624. doi:10.1083/jcb.200205091
Gellerich FN, Gizatullina Z, Trumbeckaite S, Nguyen HP, Pallas T, Arandarcikaite O, Vielhaber S, Seppet E, Striggow F (2010) The regulation of OXPHOS by extramitochondrial calcium. Biochim Biophys Acta 1797(6–7):1018–1027. doi:10.1016/j.bbabio.2010.02.005
Bononi A, Bonora M, Marchi S, Missiroli S, Poletti F, Giorgi C, Pandolfi PP, Pinton P (2013) Identification of PTEN at the ER and MAMs and its regulation of Ca(2+) signaling and apoptosis in a protein phosphatase-dependent manner. Cell Death Differ 20(12):1631–1643. doi:10.1038/cdd.2013.77
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397(6716):271–274. doi:10.1038/16729
Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D (1998) Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J 17(19):5708–5717. doi:10.1093/emboj/17.19.5708
Shen J, Chen X, Hendershot L, Prywes R (2002) ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 3(1):99–111
Schröder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74(1):739–789. doi:10.1146/annurev.biochem.73.011303.074134
BP T, Weissman JS (2004) Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol 164(3):341–346. doi:10.1083/jcb.200311055
Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J, Iglewski M, Chiong M, Simmen T, Zorzano A, Hill JA, Rothermel BA, Szabadkai G, Lavandero S (2011) Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci 124(Pt 13):2143–2152. doi:10.1242/jcs.080762
Munoz JP, Ivanova S, Sánchez-Wandelmer J, Martínez-Cristóbal P, Noguera E, Sancho A, Díaz-Ramos A, Hernández-Alvarez MI, Sebastián D, Mauvezin C, Palacín M, Zorzano A (2013) Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J 32(17):2348–2361. doi:10.1038/emboj.2013.168
Lièvremont JP, Rizzuto R, Hendershot L, Meldolesi J (1997) BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+. J Biol Chem 272(49):30873–30879
Lynes EM, Bui M, Yap MC, Benson MD, Schneider B, Ellgaard L, Berthiaume LG, Simmen T (2012) Palmitoylated TMX and calnexin target to the mitochondria-associated membrane. EMBO J 31(2):457–470. doi:10.1038/emboj.2011.384
Gilady SY, Bui M, Lynes EM, Benson MD, Watts R, Vance JE, Simmen T (2010) Ero1alpha requires oxidizing and normoxic conditions to localize to the mitochondria-associated membrane (MAM). Cell Stress and Chaperones 15(5):619–629. doi:10.1007/s12192-010-0174-1
Anelli T, Bergamelli L, Margittai E, Rimessi A, Fagioli C, Malgaroli A, Pinton P, Ripamonti M, Rizzuto R, Sitia R (2012) Ero1α regulates Ca(2+) fluxes at the endoplasmic reticulum-mitochondria interface (MAM). Antioxidants and Redox Signaling 16(10):1077–1087. doi:10.1089/ars.2011.4004
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225. doi:10.1038/nature09663
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140(6):821–832. doi:10.1016/j.cell.2010.01.040 S0092-8674(10)00075-9
Franchi L, Munoz-Planillo R, Reimer T, Eigenbrod T, Nunez G (2010) Inflammasomes as microbial sensors. Eur J Immunol 40(3):611–615. doi:10.1002/eji.200940180
Jin C, Flavell RA (2010) Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol 30(5):628–631. doi:10.1007/s10875-010-9440-3
Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320(5876):674–677. doi:10.1126/science.1156995
Zhang X, Zhang JH, Chen XY, Hu QH, Wang MX, Jin R, Zhang QY, Wang W, Wang R, Kang LL, Li JS, Li M, Pan Y, Huang JJ, Kong LD (2015) Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid Redox Signal 22(10):848–870. doi:10.1089/ars.2014.5868
Saxena G, Chen J, Shalev A (2010) Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem 285(6):3997–4005. doi:10.1074/jbc.M109.034421
Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5(7):730–737. doi:10.1038/ni1087
Gack MU, Shin YC, Joo C-H, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU (2007) TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446(7138):916–920. doi:10.1038/nature05732
Renauld J-C (2003) Class II cytokine receptors and their ligands: key antiviral and inflammatory modulators. Nature reviews. Immunology 3(8):667–676. doi:10.1038/nri1153
Kumar H, Kawai T, Kato H, Sato S, Takahashi K, Coban C, Yamamoto M, Uematsu S, Ishii KJ, Takeuchi O, Akira S (2006) Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med 203(7):1795–1803. doi:10.1084/jem.20060792
Dixit E, Boulant S, Zhang Y, Lee ASY, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, Nibert ML, Superti-Furga G, Kagan JC (2010) Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141(4):668–681. doi:10.1016/j.cell.2010.04.018
Seth RB, Sun L, Ea C-K, Chen ZJ (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122(5):669–682. doi:10.1016/j.cell.2005.08.012
Horner SM, Liu HM, Park HS, Briley J, Gale M (2011) Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc Natl Acad Sci U S A 108(35):14590–14595. doi:10.1073/pnas.1110133108
Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M (2008) Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454(7203):523–527. doi:10.1038/nature07106
Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J (2005) Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437(7062):1167–1172. doi:10.1038/nature04193
Horner SM (2014) Activation and evasion of antiviral innate immunity by hepatitis C virus. J Mol Biol 426(6):1198–1209. doi:10.1016/j.jmb.2013.10.032
Horner SM, Wilkins C, Badil S, Iskarpatyoti J, Gale M (2015) Proteomic analysis of mitochondrial-associated ER membranes (MAM) during RNA virus infection reveals dynamic changes in protein and organelle trafficking. PLoS One 10(3):e0117963. doi:10.1371/journal.pone.0117963
Vance DE, Walkey CJ, Cui Z (1997) Phosphatidylethanolamine N-methyltransferase from liver. Biochim Biophys Acta 1348(1–2):142–150
Gaigg B, Simbeni R, Hrastnik C, Paltauf F, Daum G (1995) Characterization of a microsomal subfraction associated with mitochondria of the yeast, Saccharomyces cerevisiae. Involvement in synthesis and import of phospholipids into mitochondria. Biochim Biophys Acta 1234(2):214–220
Stone SJ, Levin MC, Zhou P, Han J, Walther TC, Farese RV Jr (2009) The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem 284(8):5352–5361. doi:10.1074/jbc.M805768200
Lewin TM, Kim JH, Granger DA, Vance JE, Coleman RA (2001) Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J Biol Chem 276(27):24674–24679. doi:10.1074/jbc.M102036200
Area-Gomez E, de Groof AJ, Boldogh I, Bird TD, Gibson GE, Koehler CM, WH Y, Duff KE, Yaffe MP, Pon LA, Schon EA (2009) Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria. Am J Pathol 175(5):1810–1816. doi:10.2353/ajpath.2009.090219
Jansen KL, Faull RL, Storey P, Leslie RA (1993) Loss of sigma binding sites in the CA1 area of the anterior hippocampus in Alzheimer’s disease correlates with CA1 pyramidal cell loss. Brain Res 623(2):299–302
Hyrskyluoto A, Pulli I, Tornqvist K, Ho TH, Korhonen L, Lindholm D (2013) Sigma-1 receptor agonist PRE084 is protective against mutant huntingtin-induced cell degeneration: involvement of calpastatin and the NF-kappaB pathway. Cell Death Dis 4:e646. doi:10.1038/cddis.2013.170
Al-Saif A, Al-Mohanna F, Bohlega S (2011) A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol 70(6):913–919. doi:10.1002/ana.22534
Moustaqim-Barrette A, Lin YQ, Pradhan S, Neely GG, Bellen HJ, Tsuda H (2014) The amyotrophic lateral sclerosis 8 protein, VAP, is required for ER protein quality control. Hum Mol Genet 23(8):1975–1989. doi:10.1093/hmg/ddt594
Atwal RS, Xia J, Pinchev D, Taylor J, Epand RM, Truant R (2007) Huntingtin has a membrane association signal that can modulate huntingtin aggregation, nuclear entry and toxicity. Hum Mol Genet 16(21):2600–2615. doi:10.1093/hmg/ddm217
Reijonen S, Putkonen N, Norremolle A, Lindholm D, Korhonen L (2008) Inhibition of endoplasmic reticulum stress counteracts neuronal cell death and protein aggregation caused by N-terminal mutant huntingtin proteins. Exp Cell Res 314(5):950–960. doi:10.1016/j.yexcr.2007.12.025
Mishina M, Ohyama M, Ishii K, Kitamura S, Kimura Y, Oda K, Kawamura K, Sasaki T, Kobayashi S, Katayama Y, Ishiwata K (2008) Low density of sigma1 receptors in early Alzheimer’s disease. Ann Nucl Med 22(3):151–156. doi:10.1007/s12149-007-0094-z
Mishina M, Ishiwata K, Ishii K, Kitamura S, Kimura Y, Kawamura K, Oda K, Sasaki T, Sakayori O, Hamamoto M, Kobayashi S, Katayama Y (2005) Function of sigma1 receptors in Parkinson’s disease. Acta neurologica. Scandinavica 112(2):103–107. doi:10.1111/j.1600-0404.2005.00432.x
Duennwald ML, Lindquist S (2008) Impaired ERAD and ER stress are early and specific events in polyglutamine toxicity. Genes Dev 22(23):3308–3319. doi:10.1101/gad.1673408
Kyttala A, Ihrke G, Vesa J, Schell MJ, Luzio JP (2004) Two motifs target batten disease protein CLN3 to lysosomes in transfected nonneuronal and neuronal cells. Mol Biol Cell 15(3):1313–1323. doi:10.1091/mbc.E03-02-0120
Padilla-Lopez S, Langager D, Chan CH, Pearce DA (2012) BTN1, the Saccharomyces cerevisiae homolog to the human batten disease gene, is involved in phospholipid distribution. Disease Models & Mechanisms 5(2):191–199. doi:10.1242/dmm.008490
Schon EA, Przedborski S (2011) Mitochondria: the next (neurode)generation. Neuron 70(6):1033–1053. doi:10.1016/j.neuron.2011.06.003
Ryan BJ, Hoek S, Fon EA, Wade-Martins R (2015) Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci 40(4):200–210. doi:10.1016/j.tibs.2015.02.003
Irrcher I, Aleyasin H, Seifert EL, Hewitt SJ, Chhabra S, Phillips M, Lutz AK, Rousseaux MW, Bevilacqua L, Jahani-Asl A, Callaghan S, MacLaurin JG, Winklhofer KF, Rizzu P, Rippstein P, Kim RH, Chen CX, Fon EA, Slack RS, Harper ME, McBride HM, Mak TW, Park DS (2010) Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet 19(19):3734–3746. doi:10.1093/hmg/ddq288
Wang X, Petrie TG, Liu Y, Liu J, Fujioka H, Zhu X (2012) Parkinson’s disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J Neurochem 121(5):830–839. doi:10.1111/j.1471-4159.2012.07734.x
Smith GA, Jansson J, Rocha EM, Osborn T, Hallett PJ, Isacson O (2015) Fibroblast biomarkers of sporadic Parkinson’s disease and LRRK2 kinase inhibition. Mol Neurobiol. doi:10.1007/s12035-015-9435-4
Ozcan L, Tabas I (2012) Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med 63:317–328. doi:10.1146/annurev-med-043010-144749
Gorbatyuk MS, Shabashvili A, Chen W, Meyers C, Sullivan LF, Salganik M, Lin JH, Lewin AS, Muzyczka N, Gorbatyuk OS (2012) Glucose regulated protein 78 diminishes alpha-synuclein neurotoxicity in a rat model of Parkinson disease. Molecular Therapy: the Journal of the American Society of Gene Therapy 20(7):1327–1337. doi:10.1038/mt.2012.28
Colla E, Coune P, Liu Y, Pletnikova O, Troncoso JC, Iwatsubo T, Schneider BL, Lee MK (2012) Endoplasmic reticulum stress is important for the manifestations of alpha-synucleinopathy in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience 32(10):3306–3320. doi:10.1523/JNEUROSCI.5367-11.2012
Cho HJ, Yu J, Xie C, Rudrabhatla P, Chen X, Wu J, Parisiadou L, Liu G, Sun L, Ma B, Ding J, Liu Z, Cai H (2014) Leucine-rich repeat kinase 2 regulates Sec16A at ER exit sites to allow ER-Golgi export. EMBO J 33(20):2314–2331. doi:10.15252/embj.201487807
Guardia-Laguarta C, Area-Gomez E, Rub C, Liu Y, Magrane J, Becker D, Voos W, Schon EA, Przedborski S (2014) Alpha-synuclein is localized to mitochondria-associated ER membranes. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience 34(1):249–259. doi:10.1523/JNEUROSCI.2507-13.2014
Guardia-Laguarta C, Area-Gomez E, Schon EA, Przedborski S (2015) A new role for alpha-synuclein in Parkinson’s disease: alteration of ER-mitochondrial communication. Movement Disorders: Official Journal of the Movement Disorder Society 30(8):1026–1033. doi:10.1002/mds.26239
Cali T, Ottolini D, Negro A, Brini M (2012) Alpha-synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J Biol Chem 287(22):17914–17929. doi:10.1074/jbc.M111.302794
Sun X, Liu J, Crary JF, Malagelada C, Sulzer D, Greene LA, Levy OA (2013) ATF4 protects against neuronal death in cellular Parkinson’s disease models by maintaining levels of parkin. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience 33(6):2398–2407. doi:10.1523/JNEUROSCI.2292-12.2013
Bouman L, Schlierf A, Lutz AK, Shan J, Deinlein A, Kast J, Galehdar Z, Palmisano V, Patenge N, Berg D, Gasser T, Augustin R, Trumbach D, Irrcher I, Park DS, Wurst W, Kilberg MS, Tatzelt J, Winklhofer KF (2011) Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ 18(5):769–782. doi:10.1038/cdd.2010.142
Cali T, Ottolini D, Negro A, Brini M (2013) Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca(2+) transfer to sustain cell bioenergetics. Biochim Biophys Acta 1832(4):495–508. doi:10.1016/j.bbadis.2013.01.004
Duplan E, Giaime E, Viotti J, Sevalle J, Corti O, Brice A, Ariga H, Qi L, Checler F, Alves da Costa C (2013) ER-stress-associated functional link between parkin and DJ-1 via a transcriptional cascade involving the tumor suppressor p53 and the spliced X-box binding protein XBP-1. J Cell Sci 126(Pt 9):2124–2133. doi:10.1242/jcs.127340
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299(5604):256–259. doi:10.1126/science.1077209
Yokota T, Sugawara K, Ito K, Takahashi R, Ariga H, Mizusawa H (2003) Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem Biophys Res Commun 312(4):1342–1348
Ottolini D, Cali T, Negro A, Brini M (2013) The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum-mitochondria tethering. Hum Mol Genet 22(11):2152–2168. doi:10.1093/hmg/ddt068
Guardia-Laguarta C, Area-Gomez E, Schon EA, Przedborski S (2015) Novel subcellular localization for alpha-synuclein: possible functional consequences. Front Neuroanat 9:17. doi:10.3389/fnana.2015.00017
Acknowledgments
M. R.-A. was supported by a FPU predoctoral fellowship (FPU13/01237) from Ministerio de Educación, Cultura y Deporte, Spain. P.G-S. was funded by Parkinson’s UK. R. G.-S. was supported by a Marie Sklodowska-Curie Individual Fellowship (IF-EF) from the European Commission. J.M. B-SP was funded by La Ligue contre le Cancer. J. M. F. received research support from the Ministerio de Economia y Competitividad, Spain, CIBERNED (CB06/05/004 and PI15/00034. R. A. G.-P. was supported by a “Contrato destinado a la retención y atracción del talento investigador, TA13009” from Junta de Extremadura, Spain, and she received research support from Ministerio de Economía y Competitividad, Spain (PI14/00170). M. N-S was supported by “Contrato Juan de la Cierva” (JCI-2012-14383) from Ministerio de Economia y Competitividad, Spain. This work was supported also by “Fondo Europeo de Desarrollo Regional” (FEDER), from European Union. The authors also thank FUNDESALUD for helpful assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
M. Rodríguez-Arribas, S. M. S. Yakhine-Diop contributed equally.
Rights and permissions
About this article
Cite this article
Rodríguez-Arribas, M., Yakhine-Diop, S.M.S., Pedro, J.M.BS. et al. Mitochondria-Associated Membranes (MAMs): Overview and Its Role in Parkinson’s Disease. Mol Neurobiol 54, 6287–6303 (2017). https://doi.org/10.1007/s12035-016-0140-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0140-8