Abstract
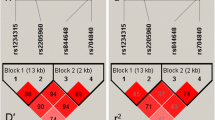
The CD40 gene is associated with many autoimmune diseases; however, there are few studies in literatures that investigate the association between CD40 and neuromyelitis optica spectrum disorders (NMOSD). This study aimed to estimate the potential association of CD40 gene polymorphisms with susceptibility to NMOSD. Four SNPs (rs1883832, rs3765459, rs4810485, and rs6074022) were selected and genotyped in a Chinese cohort comprising 162 patients with NMOSD and 237 healthy controls. P values, odds ratios (ORs), and 95 % confidential intervals (CI) for four test models (allelic, additive, dominant, and recessive) were used to assess relationships between CD40 and NMOSD. Results showed that the rs3765459 variant was significantly associated with increased risk of NMOSD in allelic model (OR = 1.48, 95 % CI 1.10–1.98, P = 0.009, P corr = 0.037), and similar results were detected in the additive and recessive models (OR = 1.47, 95 % CI 1.09–1.97, P = 0.010, P corr = 0.042; OR = 2.12, 95 % CI 1.18–3.8, P = 0.012, P corr = 0.048, respectively). Other three SNPs showed protections on NMOSD in dominant models (rs6074022, OR = 0.64, 95 % CI 0.42–0.95, P = 0.031; rs1883832, OR = 0.65, 95 % CI 0.43–0.97, P = 0.036; and rs4810485, OR = 0.63, 95 % CI 0.42–0.95, P = 0.029, respectively), but not significantly after Bonferroni corrections for multiple tests. In addition, haplotype analysis of these SNPs in tight linkage did not reveal significant association with NMOSD. This study indicates that the rs3765459 variant in CD40 gene is associated with susceptibility to NMOSD. Larger sample size studies in other ethnicities are needed to verify this association.

Similar content being viewed by others
References
Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66(10):1485–1489
Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG (2007) The spectrum of neuromyelitis optica. Lancet Neurol 6(9):805–815
Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364(9451):2106–2112
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, et al. (2015) International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85(2):177–189
Ratelade J, Verkman AS (2012) Neuromyelitis optica: aquaporin-4 based pathogenesis mechanisms and new therapies. Int J Biochem Cell Biol 44(9):1519–1530
Pittock SJ, Lennon VA, Wingerchuk DM (2006) The prevalence of non-organ-specific autoantibodies and NMO-IgG in neuromyelitis optica (NMO) and related disorders. Neurology 66(5):307
Kim JY, Bae JS, Kim HJ, et al. (2014) CD58 polymorphisms associated with the risk of neuromyelitis optica in a Korean population. BMC Neurol 14(1):57
Wang X, Yu T, Yan Q, Wang W2, Meng N, Li X, Luo Y (2016) Significant association between Fc receptor-like 3 polymorphisms (-1901A>G and -658C>T) and neuromyelitis optica (NMO) susceptibility in the Chinese population. Mol Neurobiol 53(1):686–694
Paulie S, Ehlin-Henriksson B, Mellstedt H, Koho H, Ben-Aissa H, Perlmann P (1985) A p50 surface antigen restricted to human urinary bladder carcinomas and B lymphocytes. Cancer Immunol Immunother 20(1):23–28
Bishop GA (2009) The many faces of CD40: multiple roles in normal immunity and disease. Semin Immunol 2009 21(5):255–256
Chen JM, Guo J, Wei CD, Wang CF, Luo HC, Wei YS, Lan Y (2015) The association of CD40 polymorphisms with CD40 serum levels and risk of systemic lupus erythematosus. BMC Genet 16(1):1
Joo YB, Park BL, Shin HD, Park SY, Kim I, Bae SC (2013) Association of genetic polymorphisms in CD40 with susceptibility to SLE in the Korean population. Rheumatology 52(4):623–630
Van der Linden MPM, Feitsma AL, le Cessie S, Kern M, Olsson LM, Raychaudhuri S, Begovich AB, Chang M, et al. (2009) Association of a single-nucleotide polymorphism in CD40 with the rate of joint destruction in rheumatoid arthritis. Arthritis Rheum 60(8):2242–2247
Casiraghi C, Márquez AC, Shanina I, Horowitz MS (2015) Latent virus infection upregulates CD40 expression facilitating enhanced autoimmunity in a model of multiple sclerosis. Sci Rep 5:13995
Blanco-Kelly F, Matesanz F, Alcina A, Teruel M, Díaz-Gallo LM, Gómez-García M, López-Nevot MA, Rodrigo L, et al. (2010) CD40: novel association with Crohn’s disease and replication in multiple sclerosis susceptibility. PLoS One 5(7):e11520
Kim TY, Park YJ, Hwang JK, Song JY, Park KS, Cho BY, Park DJ (2003) A C/T polymorphism in the 5′-untranslated region of the CD40 gene is associated with Graves’ disease in Koreans. Thyroid 13(10):919–925
Bahlo M, Booth DR, Broadley SA, et al., Australia and New Zealand Multiple Sclerosis Genetics Consortium (ANZgene) (2009) Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet 41(7):824-828.
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis an expanded disability status scale (EDSS). Neurology 33(11):1444–1452
Shi YY, He L (2005) SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res 15(2):97–98
Stout RD, Suttles J (1996) The many roles of CD40 in cell-mediated inflammatory responses. Immunol Today 17(10):487–492
Bishop GA, Hostager BS (2003) The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev 14(3):297–309
Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M (2009) CD40–CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proceedings of the National Academy of Sciences 106(3):876–881
Gutierrez-Arcelus M, Rich SS, Raychaudhuri S (2016) Autoimmune diseases—connecting risk alleles with molecular traits of the immune system [J]. Nat Rev Genet 17(3):160–174
Varrin-Doyer M, Spencer CM, Schulze-Topphoff U, Nelson PA, Stroud RM, Cree BA, Zamvil SS (2012) Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize clostridium ABC transporter. Ann Neurol 72(1):53–64
Gerritse K, Laman JD, Noelle RJ, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E (1996) CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis [J]. Proc Natl Acad Sci U S A 93(6):2499–2504
Mach F, Schönbeck U, Sukhova GK, Atkinson E, Libby P (1998) Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature 394(6689):200–203
Durie FH, Fava RA, Foy TM, Aruffo A, Ledbetter JA, Noelle RJ (1993) Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science 261:1328–1328
Mohan C, Shi Y, Laman JD, Datta SK (1995) Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J Immunol 154:1470–1480
Carayanniotis G, Master SR, Noelle RJ (1997) Suppression of murine thyroiditis via blockade of the CD40-CD40L interaction. Immunity 90:421–426
Ärlestig L, Rantapää-Dahlqvist S (2012) Polymorphisms of the genes encoding CD40 and growth differentiation factor 15 and in the 9p21.3 region in patients with rheumatoid arthritis and cardiovascular disease. J Rheumatol 39(5):939–945
Raychaudhuri S, Remmers EF, Lee AT, et al. (2008) Common variants at CD40 and other loci confer risk of rheumatoid arthritis [J]. Nat Genet 40(10):1216–1223
Orozco G, Eyre S, Hinks A, et al. (2010) Association of CD40 with rheumatoid arthritis confirmed in a large UK case-control study [J]. Ann Rheum Dis 69(5):813–816
Vazgiourakis V M, Zervou M I, Choulaki C, et al.(2011) A common SNP in the CD40 region is associated with systemic lupus erythematosus and correlates with altered CD40 expression: implications for the pathogenesis [J]. Annals of the Rheumatic Diseases, annrheumdis146530.
Lee HS, Korman BD, Le JM, et al. (2009) Genetic risk factors for rheumatoid arthritis differ in Caucasian and Korean populations [J]. Arthritis & Rheumatism 60(2):364–371
Jacobson EM, Concepcion E, Oashi T, Tomer Y (2005) A Graves’ disease-associated Kozak sequence single-nucleotide polymorphism enhances the efficiency of CD40 gene translation: a case for translational pathophysiology. Endocrinology 146(6):2684–2691
Wang PW, Chen IY, Juo SH, Hsi E, Liu RT, Hsieh CJ (2012) Genotype and phenotype predictors of relapse of Graves’ disease after antithyroid drug withdrawal. Eur Thyroid J 1(4):251–258
Lee HJ, Li CW, Hammerstad SS, Stefan M, Tomer Y (2015) Immunogenetics of autoimmune thyroid diseases: a comprehensive review. J Autoimmun 64:82–90
Gandhi K S, McKay F C, Cox M, et al. (2010) The multiple sclerosis whole blood mRNA transcriptome and genetic associations indicate dysregulation of specific T cell pathways in pathogenesis [J]. Human molecular genetics, ddq090.
Acknowledgments
We are grateful to the subjects for their participation and to Editage (http:// online. editage.cn/) for English language editing. We would also like to thank Dr. Yu Tao (Genesky Biotechnologies Inc., Shanghai, China) for assisting with the statistical analysis and Dr. Xinglong Yang (Sichuan University, Chengdu, China) for his revision comments. The study was funded by the National Natural Science Foundation of China (Grant No. 81271321) and the Department of Science and Technology Research Projects in the Sichuan Province of China (Grant No. 2013FZ0015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Medical Ethics Committee of the West China Hospital, Sichuan University, and performed in accordance with the ethical standards of the Declaration of Helsinki. All participants gave their informed consent prior to their inclusion in this study.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Ziyan Shi and Qin Zhang were the first co-authors due to their equal contributions to this work.
Rights and permissions
About this article
Cite this article
Shi, Z., Zhang, Q., Chen, H. et al. Association of CD40 Gene Polymorphisms with Susceptibility to Neuromyelitis Optica Spectrum Disorders. Mol Neurobiol 54, 5236–5242 (2017). https://doi.org/10.1007/s12035-016-0070-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0070-5