Abstract
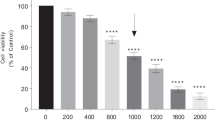
Inhibition of nitric oxide synthases (NOSs) shows promise to halt the progression of neurodegenerative diseases. The present study was designed to explore whether botanical isoflavone puerarin could attenuate nitric oxide (NO)-mediated neurotoxicity via modulating the enzymes in the L-arginine–NO pathway. Neurotoxin 6-hydroxydopamine (6-OHDA) is well known to induce neurodegeneration via a NO-dependent mechanism. We first validated that puerarin protected rat dopamingeric PC12 cells against 6-OHDA-induced neurotoxicity in a concentration-dependent manner. We subsequently profiled the cellular responses to puerarin by a proteomic response fingerprinting approach. A total of 16 protein spots with >1.5-fold change of intensity were selected and identified by mass spectrometry. As one of puerarin-upregulated proteins, mitochondrial arginase-2 hydrolyzes L-arginine to L-ornithine, thereby competing with neuronal NOS for substrate L-arginine in mitochondria. Thus, we hypothesize that puerain may attenuate nitric oxide (NO)-mediated mitochondrial injury via increasing arginase-2 expression. Western blot and reverse transcription polymerase chain reaction (RT-PCR) analyses confirmed that puerarin increased arginase-2 expression in a concentration- and time-dependent manner. Accordingly, puerarin suppressed 6-OHDA-induced NO production and neurotoxicity in PC12 cells and primary rat midbrain neurons. Arginase inhibitor BEC diminished the effect of puerarin on 6-OHDA-induced NO production and neurotoxicity. The activation of arginase-2 by puerarin represents an endogenous mechanism for specific control of NO-mediated mitochondrial damage. Thus, puerarin is a useful lead for suppressing NO-mediated neurotoxicity in neurodegenerative diseases.

Arginase-2 dependent mechanism underlying the neuroprotective activity of puerarin






Similar content being viewed by others
References
Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8(10):766–775
Virarkar M, Alappat L, Bradford PG, Awad AB (2013) L-arginine and nitric oxide in CNS function and neurodegenerative diseases. Crit Rev Food Sci Nutr 53(11):1157–1167
Luth HJ, Munch G, Arendt T (2002) Aberrant expression of NOS isoforms in Alzheimer’s disease is structurally related to nitrotyrosine formation. Brain Res 953(1–2):135–143
Daff S (2010) NO synthase: structures and mechanisms. Nitric Oxide 23(1):1–11
Zhou L, Zhu DY (2009) Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 20(4):223–230
Malinski T (2007) Nitric oxide and nitroxidative stress in Alzheimer’s disease. J Alzheimers Dis 11(2):207–218
Luiking YC, Engelen MP, Deutz NE (2010) Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care 13(1):97–104
Hantraye P, Brouillet E, Ferrante R, Palfi S, Dolan R, Matthews RT et al (1996) Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat Med 2(9):1017–1021
Silverman RB (2009) Design of selective neuronal nitric oxide synthase inhibitors for the prevention and treatment of neurodegenerative diseases. Acc Chem Res 42(3):439–451
Ebadi M, Sharma SK (2003) Peroxynitrite and mitochondrial dysfunction in the pathogenesis of Parkinson’s disease. Antioxid Redox Signal 5(3):319–335
Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS (2002) Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci 962:318–331
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336(Pt 1):1–17
Cederbaum SD, Yu H, Grody WW, Kern RM, Yoo P, Iyer RK (2004) Arginases I and II: do their functions overlap? Mol Genet Metab 81(Suppl 1):S38–S44
Mori M, Gotoh T (2004) Arginine metabolic enzymes, nitric oxide and infection. J Nutr 134(10 Suppl):2820S–2825S, discussion 53S
Morris SM Jr (2009) Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol 157(6):922–930
Bambrick LL, Yarowsky PJ, Krueger BK (1995) Glutamate as a hippocampal neuron survival factor: an inherited defect in the trisomy 16 mouse. Proc Natl Acad Sci U S A 92(21):9692–9696
Chen LW, Wang YQ, Wei LC, Shi M, Chan YS (2007) Chinese herbs and herbal extracts for neuroprotection of dopaminergic neurons and potential therapeutic treatment of Parkinson’s disease. CNS Neurol Disord Drug Targets 6(4):273–281
More SV, Kumar H, Kang SM, Song SY, Lee K, Choi DK (2013) Advances in neuroprotective ingredients of medicinal herbs by using cellular and animal models of Parkinson’s disease. Evid Based Complement Alternat Med 2013:957875
Gundimeda U, McNeill TH, Schiffman JE, Hinton DR, Gopalakrishna R (2010) Green tea polyphenols potentiate the action of nerve growth factor to induce neuritogenesis: possible role of reactive oxygen species. J Neurosci Res 88(16):3644–3655
Spencer JP (2010) The impact of fruit flavonoids on memory and cognition. Br J Nutr 104(Suppl 3):S40–S47
Zhou YX, Zhang H, Peng C (2014) Puerarin: a review of pharmacological effects. Phytother Res 28(7):961–975
Zhang X, Xiong J, Liu S, Wang L, Huang J, Liu L et al (2014) Puerarin protects dopaminergic neurons in Parkinson’s disease models. Neuroscience 280:88–98
Zhu G, Wang X, Wu S, Li Q (2012) Involvement of activation of PI3K/Akt pathway in the protective effects of puerarin against MPP+-induced human neuroblastoma SH-SY5Y cell death. Neurochem Int 60(4):400–408
Zhao J, Cheng YY, Fan W, Yang CB, Ye SF, Cui W et al (2014) Botanical drug puerarin coordinates with nerve growth factor in the regulation of neuronal survival and neuritogenesis via activating ERK1/2 and PI3K/Akt signaling pathways in the neurite extension process. CNS Neurosci Ther
Zhou Y, Xie N, Li L, Zou Y, Zhang X, Dong M (2014) Puerarin alleviates cognitive impairment and oxidative stress in APP/PS1 transgenic mice. Int J Neuropsychopharmacol 17(4):635–644
Qi H, Zhao J, Han Y, Lau AS, Rong J (2012) Z-ligustilide potentiates the cytotoxicity of dopamine in rat dopaminergic PC12 cells. Neurotox Res
Sanders LH, McCoy J, Hu X, Mastroberardino PG, Dickinson BC, Chang CJ et al (2014) Mitochondrial DNA damage: molecular marker of vulnerable nigral neurons in Parkinson’s disease. Neurobiol Dis 70:214–223
Qi H, Wei L, Han Y, Zhang Q, Lau AS, Rong J (2010) Proteomic characterization of the cellular response to chemopreventive triterpenoid astragaloside IV in human hepatocellular carcinoma cell line HepG2. Int J Oncol 36(3):725–735
Qi H, Chen B, Le XC, Rong J (2012) Concomitant induction of heme oxygenase-1 attenuates the cytotoxicity of arsenic species from lumbricus extract in human liver HepG2 cells. Chem Biodivers 9(4):739–754
Qi H, Han Y, Rong J (2012) Potential roles of PI3K/Akt and Nrf2-Keap1 pathways in regulating hormesis of Z-ligustilide in PC12 cells against oxygen and glucose deprivation. Neuropharmacology 62(4):1659–1670
Wei L, Liu J, Le XC, Han Y, Tong Y, Lau AS et al (2011) Pharmacological induction of leukotriene B4-12-hydroxydehydrogenase suppresses the oncogenic transformation of human hepatoma HepG2 cells. Int J Oncol 39(3):735–745
Tao L, Li X, Zhang L, Tian J, Li X, Sun X et al (2011) Protective effect of tetrahydroxystilbene glucoside on 6-OHDA-induced apoptosis in PC12 cells through the ROS-NO pathway. PLoS One 6(10), e26055
Cui W, Zhang Z, Li W, Hu S, Mak S, Zhang H et al (2013) The anti-cancer agent SU4312 unexpectedly protects against MPP(+) -induced neurotoxicity via selective and direct inhibition of neuronal NOS. Br J Pharmacol 168(5):1201–1214
Pulichino AM, Wang IM, Caron A, Mortimer J, Auger A, Boie Y et al (2008) Identification of transforming growth factor beta1-driven genetic programs of acute lung fibrosis. Am J Respir Cell Mol Biol 39(3):324–336
Lu MJ, Chen YS, Huang HS, Ma MC (2012) Erythropoietin alleviates post-ischemic injury of rat hearts by attenuating nitrosative stress. Life Sci 90(19–20):776–784
Cheng Y, Yang C, Zhao J, Tse HF, Rong J (2014) Proteomic identification of calcium-binding chaperone calreticulin as a potential mediator for the neuroprotective and neuritogenic activities of fruit-derived glycoside amygdalin. J Nutr Biochem
Rong J, Cheung CY, Lau AS, Shen J, Tam PK, Cheng YC (2008) Induction of heme oxygenase-1 by traditional Chinese medicine formulation ISF-1 and its ingredients as a cytoprotective mechanism against oxidative stress. Int J Mol Med 21(4):405–411
Pastwa E, Somiari SB, Czyz M, Somiari RI (2007) Proteomics in human cancer research. Proteomics Clin Appl 1(1):4–17
Oh JE, Karlmark Raja K, Shin JH, Pollak A, Hengstschlager M, Lubec G (2006) Cytoskeleton changes following differentiation of N1E-115 neuroblastoma cell line. Amino Acids 31(3):289–298
Choi SY, Choi BH, Suh BC, Chae HD, Kim JS, Shin MJ et al (2001) Potentiation of PGE(2)-mediated cAMP production during neuronal differentiation of human neuroblastoma SK-N-BE(2)C cells. J Neurochem 79(2):303–310
Nakatani Y, Hokonohara Y, Tajima Y, Kudo I, Hara S (2011) Involvement of the constitutive prostaglandin E synthase cPGES/p23 in expression of an initial prostaglandin E2 inactivating enzyme, 15-PGDH. Prostaglandins Other Lipid Mediat 94(3–4):112–117
Gilles AM, Presecan E, Vonica A, Lascu I (1991) Nucleoside diphosphate kinase from human erythrocytes. Structural characterization of the two polypeptide chains responsible for heterogeneity of the hexameric enzyme. J Biol Chem 266(14):8784–8789
Postel EH, Berberich SJ, Flint SJ, Ferrone CA (1993) Human c-myc transcription factor PuF identified as nm23-H2 nucleoside diphosphate kinase, a candidate suppressor of tumor metastasis. Science 261(5120):478–480
Kim HS, Kim J, Jo Y, Jeon D, Cho YS (2014) Direct lineage reprogramming of mouse fibroblasts to functional midbrain dopaminergic neuronal progenitors. Stem Cell Res 12(1):60–68
Walsh D, Mohr I (2014) Coupling 40S ribosome recruitment to modification of a cap-binding initiation factor by eIF3 subunit e. Genes Dev 28(8):835–840
Han K, Kim MH, Seeburg D, Seo J, Verpelli C, Han S et al (2009) Regulated RalBP1 binding to RalA and PSD-95 controls AMPA receptor endocytosis and LTD. PLoS Biol 7(9), e1000187
Li L, Hung AC, Porter AG (2008) Secretogranin II: a key AP-1-regulated protein that mediates neuronal differentiation and protection from nitric oxide-induced apoptosis of neuroblastoma cells. Cell Death Differ 15(5):879–888
Ma J, Wu R, Zhang Q, Wu JB, Lou J, Zheng Z et al (2014) DJ-1 interacts with RACK1 and protects neurons from oxidative-stress-induced apoptosis. Biochem J 462(3):489–497
Kahle PJ, Waak J, Gasser T (2009) DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med 47(10):1354–1361
Yu S, Zuo X, Li Y, Zhang C, Zhou M, Zhang YA et al (2004) Inhibition of tyrosine hydroxylase expression in alpha-synuclein-transfected dopaminergic neuronal cells. Neurosci Lett 367(1):34–39
Marathe C, Bradley MN, Hong C, Lopez F, de Ruiz Galarreta CM, Tontonoz P et al (2006) The arginase II gene is an anti-inflammatory target of liver X receptor in macrophages. J Biol Chem 281(43):32197–32206
Toya T, Hakuno D, Shiraishi Y, Kujiraoka T, Adachi T (2014) Arginase inhibition augments nitric oxide production and facilitates left ventricular systolic function in doxorubicin-induced cardiomyopathy in mice. Physiol Rep 2(9)
Bansal V, Ochoa JB (2003) Arginine availability, arginase, and the immune response. Curr Opin Clin Nutr Metab Care 6(2):223–228
Stewart VC, Heales SJ (2003) Nitric oxide-induced mitochondrial dysfunction: implications for neurodegeneration. Free Radic Biol Med 34(3):287–303
Topal G, Brunet A, Walch L, Boucher JL, David-Dufilho M (2006) Mitochondrial arginase II modulates nitric-oxide synthesis through nonfreely exchangeable L-arginine pools in human endothelial cells. J Pharmacol Exp Ther 318(3):1368–1374
Acknowledgments
The authors are grateful to Miss Zhaohui Liang for her excellent technical assistance. This work was supported by General Research Fund (GRF) (HKU 775812 M) from the Research Grants Council of Hong Kong and the Seed Funding for Basic Research Programme, The University of Hong Kong.
Conflict of Interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, J., Cheng, Y., Yang, C. et al. Botanical Drug Puerarin Attenuates 6-Hydroxydopamine (6-OHDA)-Induced Neurotoxicity via Upregulating Mitochondrial Enzyme Arginase-2. Mol Neurobiol 53, 2200–2211 (2016). https://doi.org/10.1007/s12035-015-9195-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9195-1