Abstract
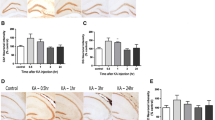
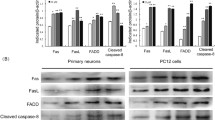
The Fas receptor (FasR)/Fas ligand (FasL) system plays a significant role in the process of neuronal loss in neurological disorders. Thus, in the present study, we used a real-time PCR array focused apoptosis (Mouse Apoptosis RT2 PCR Array) to study the role of the Fas pathway in the apoptotic process that occurs in a kainic acid (KA) mice experimental model. In fact, significant changes in the transcriptional activity of a total of 23 genes were found in the hippocampus of wild-type C57BL/6 mice after 12 h of KA treatment compared to untreated mice. Among the up-regulated genes, we found key factors involved in the extrinsic apoptotic pathway, such as tnf, fas and fasL, and also in caspase genes (caspase -4, caspase-8 and caspase-3). To discern the importance of the FasR/FasL pathway, mice lacking the functional Fas death receptor (lpr) were also treated with KA. After 24 h of neurotoxin treatment, lpr mice exhibited a reduced number of apoptotic positive cells, determined by the terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) method in different regions of the hippocampus, when compared to wild-type mice. In addition, treatment of lpr mice with KA did not produce significant changes in the transcriptional activity of genes related to apoptosis in the hippocampus, either in the fas and fas ligand genes or in caspase-4 and caspase-8 and the executioner caspase-3 genes, as occurred in wild-type C57BL/6 mice. Thus, these data provide direct evidence that Fas signalling plays a key role in the induction of apoptosis in the hippocampus following KA treatment, making the inhibition of the death receptor pathway a potentially suitable target for excitotoxicity neuroprotection in neurological conditions such as epilepsy.





Similar content being viewed by others
References
Beal MF (1998) Excitotoxicity and nitric oxide in Parkinson’s disease pathogenesis. Ann Neurol 44(Suppl 1):S110–S114
Boll MC, Alcaraz-Zubeldia M, Rios C (2011) Medical management of Parkinson’s disease: focus on neuroprotection. Curr Neuropharmacol 9:350–359
Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL (2013) Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol 698:6–18
Ben-Ari Y, Cossart R (2000) Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci 23:580–587
Benkovic SA, O’Callaghan JP, Miller DB (2004) Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res 1024:59–76
de Lemos L, Junyent F, Verdaguer E, Folch J, Romero R, Pallàs M, Ferrer I, Auladell C, Camins A (2010) Differences in activation of ERK1/2 and p38 kinase in Jnk3 null mice following KA treatment. J Neurochem 114:1315–1322
Fisahn A (2005) Kainate receptors and rhythmic activity in neuronal networks: hippocampal gamma oscillations as a tool. J Physiol 562:65–72
Tripathi PP, Santorufo G, Brilli E, Borrelli E, Bozzi Y (2011) Kainic acid-induced seizures activate GSK-3β in the hippocampus of D2R−/− mice. Neuroreport 23:846–850
Wang Q, Yu S, Simonyi A, Sun GY, Sun AY (2005) Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol Neurobiol 31:3–16
Engel T, Hatazaki S, Tanaka K, Prehn JH, Henshall DC (2010) Deletion of Puma protects hippocampal neurons in a model of severe status epilepticus. Neuroscience 168:443–450
Engel T, Murphy BM, Hatazaki S, Jimenez-Mateos EM, Concannon CG, Woods I et al (2010) Reduced hippocampal damage and epileptic seizures after status epilepticus in mice lacking proapoptotic Puma. FASEB J 24:853–861
Crespo-Biel N, Canudas AM, Camins A, Pallàs M (2007) Kainate induces AKT, ERK and cdk5/GSK3beta pathway deregulation, phosphorylates tau protein in mouse hippocampus. Neurochem Int 50:435–442
Grabenstatter HL, Del Angel YC, Carlsen J, Wempe MF, White AM et al (2014) The effect of STAT3 inhibition on status epilepticus and subsequent spontaneous seizures in the pilocarpine model of acquired epilepsy. Neurobiol Dis 62:73–85
Koeller HB, Ross ME, Glickstein SB (2008) Cyclin D1 in excitatory neurons of the adult brain enhances kainate-induced neurotoxicity. Neurobiol Dis 31:230–241
Murphy BM, Engel T, Paucard A, Hatazaki S, Mouri G, Tanaka K et al (2010) Contrasting patterns of Bim induction and neuroprotection in Bim-deficient mice between hippocampus and neocortex after status epilepticus. Cell Death Differ 17:459–468
Verdaguer E, Jiménez A, Canudas AM, Jordà EG, Sureda FX et al (2004) Inhibition of cell cycle pathway by flavopiridol promotes survival of cerebellar granule cells after an excitotoxic treatment. J Pharmacol Exp Ther 308:609–616
Algeciras-Schimnich A, Barnhart BC, Peter ME (2002) Apoptosis-independent functions of killer caspases. Curr Opin Cell Biol 14:721–726
Galluzzi L, Blomgren K, Kroemer G (2009) Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci 10:481–494
Kumar S (2007) Caspase function in programmed cell death. Cell Death Differ 14:32–43
Le-Niculescu H, Bonfoco E, Kasuya Y, Claret FX, Green DR, Karin M (1999) Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol Cell Biol 19:751–763
Henshall DC, Bonislawski DP, Skradski SL, Lan JQ, Meller R, Simon RP (2001) Cleavage of bid may amplify caspase-8-induced neuronal death following focally evoked limbic seizures. Neurobiol Dis 8:568–580
Henshall DC, Araki T, Schindler CK, Shinoda S, Lan JQ, Simon RP (2003) Expression of death-associated protein kinase and recruitment to the tumor necrosis factor signaling pathway following brief seizures. J Neurochem 86:1260–1270
Tan Z, Levid J, Schreiber SS (2001) Increased expression of Fas (CD95/APO-1) in adult rat brain after kainate-induced seizures. Neuroreport 12:1979–1982
Dzietko M, Boos V, Sifringer M, Polley O, Gerstner B, Genz K et al (2008) A critical role for Fas/CD-95 dependent signaling pathways in the pathogenesis of hyperoxia-induced brain injury. Ann Neurol 64:664–673
Ethell DW, Buhler LA (2003) Fas ligand-mediated apoptosis in degenerative disorders of the brain. J Clin Immunol 23:363–370
Felderhoff-Mueser U, Buhrer C, Groneck P, Obladen M, Bartmann P, Heep A (2003) Soluble Fas (CD95/Apo-1), soluble Fas ligand, and activated caspase 3 in the cerebrospinal fluid of infants with posthemorrhagic and nonhemorrhagic hydrocephalus. Pediatr Res 54:659–664
Grosjean MB, Lenzlinger PM, Stahel PF, Yatsiv I, Shohami E, Trentz O et al (2007) Immunohistochemical characterization of Fas (CD95) and Fas Ligand (FasL/CD95L) expression in the injured brain: relationship with neuronal cell death and inflammatory mediators. Histol Histopathol 22:235–250
Jia J, Guan D, Zhu W, Alkayed NJ, Wang MM, Hua Z, Xu Y (2009) Estrogen inhibits Fas-mediated apoptosis in experimental stroke. Exp Neurol 215:48–52
Martin-Villalba A, Herr I, Jeremias I, Hahne M, Brandt R, Vogel J et al (1999) CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J Neurosci 19:3809–3817
Chu JL, Drappa J, Parnassa A, Elkon KB (1993) The defect in Fas mRNA expression in MRL/lpr mice is associated with insertion of the retrotransposon, ETn. J Exp Med 178:723–730
Graham EM, Sheldon RA, Flock DL, Ferriero DM, Martin LJ et al (2004) Neonatal mice lacking functional Fas death receptors are resistant to hypoxic-ischemic brain injury. Neurobiol Dis 17:89–98
Niu FN, Zhang X, Hu XM, Chen J, Chang LL, Li JW et al (2012) Targeted mutation of Fas ligand gene attenuates brain inflammation in experimental stroke. Brain Behav Immun 26:61–71
Safa AR (2012) c-FLIP, a master anti-apoptotic regulator. Exp Oncol 34:176–184
Hayley S, Crocker SJ, Smith PD, Shree T, Jackson-Lewis V, Przedborski S, Mount M et al (2004) Regulation of dopaminergic loss by Fas in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J Neurosci 24:2045–2053
Landau AM, Luk KC, Jones ML, Siegrist-Johnstone R, Young YK, Kouassi E et al (2005) Defective Fas expression exacerbates neurotoxicity in a model of Parkinson’s disease. J Exp Med 202:575–581
Mogi M, Harada M, Kondo T, Mizuno Y, Narabayashi H, Riederer P, Nagatsu T (1996) The soluble form of Fas molecule is elevated in parkinsonian brain tissues. Neurosci Lett 220:195–198
Bi FF, Xiao B, Hu YQ, Tian FF, Wu ZG, Ding L, Zhou XF (2008) Expression and localization of Fas-associated proteins following focal cerebral ischemia in rats. Brain Res 1191:30–38
Ethell DW, Kinloch R, Green DR (2002) Metalloproteinase shedding of Fas ligand regulates beta-amyloid neurotoxicity. Curr Biol 12:1595–1600
Millet P, Lages CS, Haïk S, Nowak E, Allemand I, Granotier C, Boussin FD (2005) Amyloid-beta peptide triggers Fas-independent apoptosis and differentiation of neural progenitor cells. Neurobiol Dis 19:57–65
Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H et al (2001) Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci 21:7551–7560
Shah RS, Lee HG, Xiongwei Z, Perry G, Smith MA, Castellani RJ (2008) Current approaches in the treatment of Alzheimer’s disease. Biomed Pharmacother 62:199–207
Puri V, Ranjit S, Konda S, Nicoloro SM, Straubhaar J, Chawla A et al (2008) Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci U S A 105:7833–7838
Watanabe-Fukunaga R, Brannan CI, Itoh N, Yonehara S, Copeland NG et al (1992) The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol 148:1274–1279
Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S (1992) Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314–317
Leite JP, Garcia-Cairasco N, Cavalheiro EA (2002) New insights from the use of pilocarpine and kainate models. Epilepsy Res 50:93–103
Hartmann A, Mouatt-Prigent A, Faucheux BA, Agid Y, Hirsch EC (2002) FADD: a link between TNF family receptors and caspases in Parkinson’s disease. Neurology 58:308–310
Su JH, Anderson AJ, Cribbs DH, Tu C, Tong L et al (2003) Fas and Fas ligand are associated with neuritic degeneration in the AD brain and participate in beta-amyloid-induced neuronal death. Neurobiol Dis 12:182–193
Ferrer I, Puig B, Krupinsk J, Carmona M, Blanco R (2001) Fas and Fas ligand expression in Alzheimer’s disease. Acta Neuropathol 102:121–131
Williams CD, McGill MR, Farhood A, Jaeschke H (2013) Fas receptor-deficient lpr mice are protected against acetaminophen hepatotoxicity due to higher glutathione synthesis and enhanced detoxification of oxidant stress. Food Chem Toxicol 58:228–235
Puig B, Ferrer I (2002) Caspase-3-associated apoptotic cell death in excitotoxic necrosis of the entorhinal cortex following intraperitoneal injection of kainic acid in the rat. Neurosci Lett 321:182–186
Nohara N, Koseki T, Chen S, Wu X, Nunez G (1998) CIDE, a novel family of cell death activators with homology to the 45kDa subunit of the DNA fragmentation factor. EMBO J 17:2526–2533
Kampa KM, Bonin M, Lopez CD (2009) New insights into the expanding complexity of the tumor suppressor ASPP2. Cell Cycle 8:2871–2876
Alvarado Y (2012) The PIM kinases in hematological cancers. Expert Rev Hematol 5:81–96
Benkovic SA, O’Callaghan JP, Miller DB (2006) Regional neuropathology following kainic acid intoxication in adult and aged C57BL/6J mice. Brain Res 1070:215–231
Chuang YC, Lin JW, Chen SD, Lin TK, Liou CW, Lu CH, Chang WN (2009) Preservation of mitochondrial integrity and energy metabolism during experimental status epilepticus leads to neuronal apoptotic cell death in the hippocampus of the rat. Seizure 18:420–428
Diwakarla S, Nagley P, Hughes ML, Chen B, Beart PM (2009) Differential insult-dependent recruitment of the intrinsic mitochondrial pathway during neuronal programmed cell death. Cell Mol Life Sci 66:156–172
Engel T, Henshall DC (2009) Apoptosis, Bcl-2 family proteins and caspases: the ABCs of seizure-damage and epileptogenesis? Int J Physiol Pathophysiol Pharmacol 1:97–115
Wei XW, Yan H, Xu B, Wu YP, Li C, Zhang GY (2012) Neuroprotection of co-activation of GABA receptors by preventing caspase-3 denitrosylation in KA-induced seizures. Brain Res Bull 88:617–623
Torres-Peraza JF, Engel T, Martín-Ibáñez R, Sanz-Rodríguez A, Fernández-Fernández MR et al (2013) Protective neuronal induction of ATF5 in endoplasmic reticulum stress induced by status epilepticus. Brain 136:1161–1176
Wang H, Lin G, Zhang Z (2007) ATF5 promotes cell survival through transcriptional activation of Hsp27 in H9c2 cells. Cell Biol Int 31:1309–1315
Dluzen D, Li G, Tacelosky D, Moreau M, Liu DX (2011) BCL-2 is a downstream target of ATF5 that mediates the prosurvival function of ATF5 in a cell type-dependent manner. J Biol Chem 286:7705–7713
Mori M, Burgess DL, Gefrides LA, Foreman PJ, Opferman JT, Korsmeyer SJ et al (2004) Expression of apoptosis inhibitor protein Mcl1 linked to neuroprotection in CNS neurons. Cell Death Differ 11:1223–1233
Krajewska M, You Z, Rong J, Kress C, Huang X et al (2011) Neuronal deletion of caspase 8 protects against brain injury in mouse models of controlled cortical impact and kainic acid-induced excitotoxicity. PLoS ONE 6:e24341
Acknowledgments
This work was supported by Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CiberNed-Instituto de Salud Carlos III) and by grants from Ministerio de Ciencia (MICINN, MINECO) SAF2009-08233 and SAF2012-34177 and Fundación Ramón Areces to JJL. Grant 2009/SGR00853 from the Generalitat de Catalunya (Autonomous Government of Catalonia) and grants BFU2010-19119/BFI to CA, SAF2011-23631 to AC, and SAF2012-39852-C02-01 to MP from the Spanish Ministerio de Ciencia (MICINN, MINECO) also supported the study. Grant 0177594 from CONACYT (Mexico) was awarded to CBZ.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Carme Auladell and Antoni Camins are senior co-authors.
Miren Ettcheto and Felix Junyent contributed equally to the manuscript
Rights and permissions
About this article
Cite this article
Ettcheto, M., Junyent, F., de Lemos, L. et al. Mice Lacking Functional Fas Death Receptors Are Protected from Kainic Acid-Induced Apoptosis in the Hippocampus. Mol Neurobiol 52, 120–129 (2015). https://doi.org/10.1007/s12035-014-8836-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8836-0