Abstract
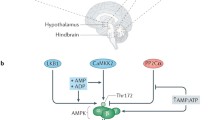
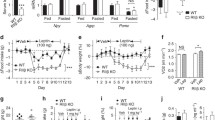
The complications caused by overweight, obesity and type 2 diabetes are one of the main problems that increase morbidity and mortality in developed countries. Hypothalamic metabolic sensors play an important role in the control of feeding and energy homeostasis. PAS kinase (PASK) is a nutrient sensor proposed as a regulator of glucose metabolism and cellular energy. The role of PASK might be similar to other known metabolic sensors, such as AMP-activated protein kinase (AMPK) and the mammalian target of rapamycin (mTOR). PASK-deficient mice resist diet-induced obesity. We have recently reported that AMPK and mTOR/S6K1 pathways are regulated in the ventromedial and lateral hypothalamus in response to nutritional states, being modulated by anorexigenic glucagon-like peptide-1 (GLP-1)/exendin-4 in lean and obese rats. We identified PASK in hypothalamic areas, and its expression was regulated under fasting/re-feeding conditions and modulated by exendin-4. Furthermore, PASK-deficient mice have an impaired activation response of AMPK and mTOR/S6K1 pathways. Thus, hypothalamic AMPK and S6K1 were highly activated under fasted/re-fed conditions. Additionally, in this study, we have observed that the exendin-4 regulatory effect in the activity of metabolic sensors was lost in PASK-deficient mice, and the anorexigenic properties of exendin-4 were significantly reduced, suggesting that PASK could be a mediator in the GLP-1 signalling pathway. Our data indicated that the PASK function could be critical for preserving the nutrient effect on AMPK and mTOR/S6K1 pathways and maintain the regulatory role of exendin-4 in food intake. Some of the antidiabetogenic effects of exendin-4 might be modulated through these processes.







Similar content being viewed by others
References
Hao HX, Rutter J (2008) The role of PAS kinase in regulating energy metabolism. IUBMB Life 60(4):204–209
Schlafli P, Borter E, Spielmann P, Wenger RH (2009) The PAS-domain kinase PASKIN: a new sensor in energy homeostasis. Cell Mol Life Sci 66(5):876–883
MacDonald PE, Rorsman P (2011) Per-arnt-sim (PAS) domain kinase (PASK) as a regulator of glucagon secretion. Diabetologia 54(4):719–721
Smith TL, Rutter J (2007) Regulation of glucose partitioning by PAS kinase and Ugp1 phosphorylation. Mol Cell 26(4):491–499
Grose JH, Smith TL, Sabic H, Rutter J (2007) Yeast PAS kinase coordinates glucose partitioning in response to metabolic and cell integrity signaling. Embo J 26(23):4824–4830
Katschinski DM, Marti HH, Wagner KF, Shibata J, Eckhardt K, Martin F, Depping R, Paasch U, Gassmann M, Ledermann B, Desbaillets I, Wenger RH (2003) Targeted disruption of the mouse PAS domain serine/threonine kinase PASKIN. Mol Cell Biol 23(19):6780–6789
Hao HX, Cardon CM, Swiatek W, Cooksey RC, Smith TL, Wilde J, Boudina S, Abel ED, McClain DA, Rutter J (2007) PAS kinase is required for normal cellular energy balance. Proc Natl Acad Sci U S A 104(39):15466–15471
da Silva XG, Farhan H, Kim H, Caxaria S, Johnson P, Hughes S, Bugliani M, Marselli L, Marchetti P, Birzele F, Sun G, Scharfmann R, Rutter J, Siniakowicz K, Weir G, Parker H, Reimann F, Gribble FM, Rutter GA (2011) Per-arnt-sim (PAS) domain-containing protein kinase is downregulated in human islets in type 2 diabetes and regulates glucagon secretion. Diabetologia 54(4):819–827
Rutter GA, Da Silva XG, Leclerc I (2003) Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem J 375(Pt 1):1–16
Hardie DG, Carling D, Carlson M (1998) The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem 67:821–855
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13(4):251–262
Foster KG, Fingar DC (2010) Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem 285(19):14071–14077
Alessi DR, Pearce LR, Garcia-Martinez JM (2009) New insights into mTOR signaling: mTORC2 and beyond. Sci Signal 2(67):pe27
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12(1):21–35
Proud CG (2002) Regulation of mammalian translation factors by nutrients. Eur J Biochem 269(22):5338–5349
Gingras AC, Raught B, Sonenberg N (2001) Regulation of translation initiation by FRAP/mTOR. Genes Dev 15(7):807–826
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115(5):577–590
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30(2):214–226
Zheng M, Wang YH, Wu XN, Wu SQ, Lu BJ, Dong MQ, Zhang H, Sun P, Lin SC, Guan KL, Han J (2011) Inactivation of Rheb by PRAK-mediated phosphorylation is essential for energy-depletion-induced suppression of mTORC1. Nat Cell Biol 13(3):263–272
Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB (2012) p70S6 kinase phosphorylates AMPK on serine 491 to mediate Leptin’s effect on food intake. Cell Metab 16(1):104–112
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB (2004) AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428(6982):569–574
Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ (2006) Hypothalamic mTOR signaling regulates food intake. Science 312(5775):927–930
Hurtado-Carneiro V, Roncero I, Blazquez E, Alvarez E, Sanz C (2013) PAS kinase as a nutrient sensor in neuroblastoma and hypothalamic cells required for the normal expression and activity of other cellular nutrient and energy sensors. Mol Neurobiol 48(3):904–920
Rodriquez de Fonseca F, Navarro M, Alvarez E, Roncero I, Chowen JA, Maestre O, Gomez R, Munoz RM, Eng J, Blazquez E (2000) Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism 49(6):709–717
Hurtado-Carneiro V, Sanz C, Roncero I, Vazquez P, Blazquez E, Alvarez E (2012) Glucagon-like peptide 1 (GLP-1) can reverse AMP-activated protein kinase (AMPK) and S6 kinase (P70S6K) activities induced by fluctuations in glucose levels in hypothalamic areas involved in feeding behaviour. Mol Neurobiol 45(2):348–361
Paxinos G, Watson C (2004) The rat brain in stereotaxic coordinates. Elsevier, New York
Franklin KBJ, Paxinos G (2008) The mouse brain in stereotaxic coordinates. Elsevier, San Diego
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159
Navarro M, Rodriquez de Fonseca F, Alvarez E, Chowen JA, Zueco JA, Gomez R, Eng J, Blazquez E (1996) Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. J Neurochem 67(5):1982–1991
da Silva XG, Rutter J, Rutter GA (2004) Involvement of Per-Arnt-Sim (PAS) kinase in the stimulation of preproinsulin and pancreatic duodenum homeobox 1 gene expression by glucose. Proc Natl Acad Sci U S A 101(22):8319–8324
Borter E, Niessen M, Zuellig R, Spinas GA, Spielmann P, Camenisch G, Wenger RH (2007) Glucose-stimulated insulin production in mice deficient for the PAS kinase PASKIN. Diabetes 56(1):113–117
Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE (2002) Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes 51(7):2056–2065
Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU (2004) Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 10(7):727–733
Cota D, Matter EK, Woods SC, Seeley RJ (2008) The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci 28(28):7202–7208
Long YC, Zierath JR (2006) AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest 116(7):1776–1783
Reed AS, Unger EK, Olofsson LE, Piper ML, Myers MG Jr, Xu AW (2010) Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes 59(4):894–906
Villanueva EC, Munzberg H, Cota D, Leshan RL, Kopp K, Ishida-Takahashi R, Jones JC, Fingar DC, Seeley RJ, Myers MG Jr (2009) Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology 150(10):4541–4551
Mori H, Inoki K, Munzberg H, Opland D, Faouzi M, Villanueva EC, Ikenoue T, Kwiatkowski D, MacDougald OA, Myers MG Jr, Guan KL (2009) Critical role for hypothalamic mTOR activity in energy balance. Cell Metab 9(4):362–374
Martins L, Fernandez-Mallo D, Novelle MG, Vazquez MJ, Tena-Sempere M, Nogueiras R, Lopez M, Dieguez C (2012) Hypothalamic mTOR signaling mediates the orexigenic action of ghrelin. PLoS One 7(10):e46923
Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ (2004) AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279(13):12005–12008
Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M (2005) Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem 280(26):25196–25201
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126(5):955–968
Semplici F, Vaxillaire M, Fogarty S, Semache M, Bonnefond A, Fontes G, Philippe J, Meur G, Diraison F, Sessions RB, Rutter J, Poitout V, Froguel P, Rutter GA (2011) Human mutation within Per-Arnt-Sim (PAS) domain-containing protein kinase (PASK) causes basal insulin hypersecretion. J Biol Chem 286(51):44005–44014
Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB (2006) Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem 281(28):18933–18941
Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF (2004) Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279(2):1070–1079
Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S (2003) The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest 111(1):91–98
Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, Foretz M, Andreelli F, Ventura-Clapier R, Bertrand L (2009) AMPK: lessons from transgenic and knockout animals. Front Biosci 14:19–44
Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ (2007) AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117(8):2325–2336
Dzamko N, van Denderen BJ, Hevener AL, Jorgensen SB, Honeyman J, Galic S, Chen ZP, Watt MJ, Campbell DJ, Steinberg GR, Kemp BE (2010) AMPK beta1 deletion reduces appetite, preventing obesity and hepatic insulin resistance. J Biol Chem 285(1):115–122
Xue B, Pulinilkunnil T, Murano I, Bence KK, He H, Minokoshi Y, Asakura K, Lee A, Haj F, Furukawa N, Catalano KJ, Delibegovic M, Balschi JA, Cinti S, Neel BG, Kahn BB (2009) Neuronal protein tyrosine phosphatase 1B deficiency results in inhibition of hypothalamic AMPK and isoform-specific activation of AMPK in peripheral tissues. Mol Cell Biol 29(16):4563–4573
Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431(7005):200–205
Acknowledgments
This work was supported by grants from Spain’s Ministry of Science and Innovation—MICINN (SAF2006-0475 and SAF2009-11297), the Complutense University-Banco Santander Funding Programme for the Creation and Consolidation of Research Teams (GR58/08, GR35/10A, GR35/10B and GR42/10), Mutua Madrileña Medical Research Foundation and the IODURE project, and the CIBER for Diabetes and Associated Metabolic Disorders (CIBERDEM) of the Carlos III Health Institute (ISCIII) (Ministry of Science and Innovation). We wish to thank Marketa Zemanova for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Carmen Sanz and Elvira Alvarez contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online resource 1
(DOC 61 kb)
Online resource 2
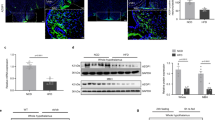
Effects of PASK deficiency on the expression of several genes in the VMH and LH. Quantitative real-time PCR was used to analyze the expression of several genes as shown (Online resource 1). The mRNA levels were quantified in VMH and LH from fasted C57Bl/6 wild-type and PASK knockout mice in the presence or absence of exendin-4. The bars represent the ratios respect to β-actin of the mRNA levels of the different genes. The value obtained in VMH from wild-type mice (WT) treated with a vehicle without exendin-4 was taken as 1. Results are means ± SEM; n=3-4 animals per condition. *P<0.05, **P<0.01 WT vs. Pask; #P<0.05 vehicle vs. exendin-4; †P<0.05, ††P<0.01 VMH vs. LH. (GIF 39 kb)
Rights and permissions
About this article
Cite this article
Hurtado-Carneiro, V., Roncero, I., Egger, S.S. et al. PAS Kinase Is a Nutrient and Energy Sensor in Hypothalamic Areas Required for the Normal Function of AMPK and mTOR/S6K1. Mol Neurobiol 50, 314–326 (2014). https://doi.org/10.1007/s12035-013-8630-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8630-4