Abstract
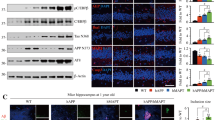
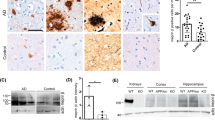
Nicastrin (NCT) is a component of the presenilin protein complex, which is involved in the cleavage of β-amyloid precursor protein (βAPP) and Notch. The aim of this study was to determine the manner in which overexpression of wild-type human nicastrin (hNCTw) or mutant human nicastrin (hNCTm, D336A/Y337A) regulates brain functions and amyloid precusor protein (APP) processing. For this, we created transgenic (Tg) mice expressing neuron-specific enolase (NSE)-controlled hNCTw or hNCTm and measured their phenotypes as time passed. The NSE/hNCTw and NSE/hNCTm Tg groups exhibited greater behavioral dysfunction from 10 months of age than the non-Tg group, although their severities differed. Further, activity and component levels of the γ-secretase complex were significantly elevated in NSE/hNCTw Tg mice, expect for PEN-2. These alterations induced stimulation of APP processing, resulting in overproduction of Aβ-42 peptide in the NSE/hNCTw Tg group, whereas the NSE/hNCTm Tg group showed a comparatively weaker effect. Furthermore, the highest expression levels of β-secretase and NICD were observed in the NSE/hNCTw Tg group, similar to other phenotypes. Especially, a significances interference on the interaction between NCT and γ-secretase substrates was detected in NSE/hNCTm Tg groups compare with NSE/hNCTw Tg group. These results indicate that hNCTw overexpression in Tg mice promoted active assembly of the γ-secretase complex through modulation of APP processing and behavior, whereas the lesser effect in NSE/hNCTm Tg mice was due to reduced expression of hNCTm. These Tg mice could be useful for the development and application of therapeutic drugs in an animal model of Alzheimer’s disease.







Similar content being viewed by others
References
Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE 3rd (2005) Nicastrin function as a γ-secretase-substrate receptor. Cell 122:435–447
Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawaral T, Supara A, Levesque L, Yu H, Yang DS, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C, Rogaev E, Smith M, Janus C, Zhang Y, Aebersold R, Farrer L, Sorbi S, Bruni A, Fraser P, St George-Hyslop P (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature 407:48–54
Ye Y, Lukinova N, Fortini ME (1999) Neurogenic phenotypes and altered Notch processing in Drosophila presenilin mutants. Nature 398:446–467
Struhl G, Greenwald I (1999) Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature 398:522–525
De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R (1999) A presenilin-1-dependent γ-secretase-loke protease mediates release of Notch intracellular domains. Nature 398:518–522
Edbauser D, Winkler E, Haass C, Steiner H (2002) Presenilin and nicastrin regulate each other and determine amyloid beta-peptide production via complex formation. Proc Natl Acad Sci USA 99:8666–8671
Kaether C, Lammich S, Edbauer D, Ertl M, Rietdorf J, Capell A, Steiner H, Haass C (2002) Presenilin-1 affects trafficking and processing of βAPP and is targeted in a complex with nicastrin to the plasma membrane. J Cell Biol 158:551–561
Kimberly WT, LaVoie MJ, Ostaszewiski BL, Ye W, Wolfe WS, Selko DJ (2002) Complex N-linked glycosylated nicastrin associates with active γ-secretase and undergoes tight cellular regulation. J Biol Chem 277:35113–35117
Leem JY, Vijayan S, Han P, Cai D, Machura D, Lopes KO, Veselits ML, Xu H, Thinakaran G (2002) Presenilin 1 is required for maturation and cell surface accumulation of nicastrin. J Biol Chem 277:19236–19240
Tomita T, Katayama R, Takikawa R, Iwatsubo T (2002) Complex N-glycosylated form of nicastrin is stabilized and selectively bound to presenilin fragments. FEBS Lett 520:117–121
Murphy MP, Das P, Nyborg AC, Rochette MJ, Dodson MW, Loosbrock NW, Soulder TM, McLendon C, Merit SL, Piper SC, Jansen KR, Golde TE (2003) Overexpression of nicastrin increases Aβ production. FASEB J 17:1138–1140
Lee SF, Shah S, Li H, Yu C, Han W, Yu G (2002) Mammalian APH-1 interact with presenilin and nicastrin and is required for intramembrane proteolysis of amyloid-β precursor protein and Notch. J Biol Chem 277:45013–45019
Li T, Ma G, Cai H, Price DL, Wong PC (2003) Nicastrin is required for assembly of presenilin/γ-secretase complexes to mediate Notch signaling and for processing and trafficking of β-amyloid precursor protein in mammals. J Neurosci 23:3272–3277
Siman R, Velji J (2003) Localization of presenilin-nicastrin complexes and gamma-secretase activity to the trans-Golgi network. J Neurochem 84:1143–1153
Morris RGM, Garrud P, Rawlins JNP, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
Tsien JZ, Huerta PT, Tonegawa S (1996) The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87:1327–1338
Prajapati KD, Sharma SS, Roy N (2010) Upregulation of albumin expression in focal ischemic rat brain. Brain Res 1327:118–1124
Torres KC, Dutra WO, Gollob KJ (2004) Endogenous IL-4 and IFN-gamma are essential for expression of Th2, but not Th1 cytokine message during the early differentiation of human CD4+ T helper cells. Hum Immunol 65:1328–1335
Hwang DY, Chae KR, Kang TS, Hwang JH, Lim CH, Kang HK, Goo JS, Lee MR, Lim HJ, Min SH, Cho JY, Hong JT, Song CW, Paik SK, Cho JS, Kim YK (2002) Alterations in behavior, amyloid β-42, caspase-3, and cox-2 in mutant PS2 Tg mouse model of Alzheimer’s disease. FASEB J 16:805–813
Hwang DY, Cho JS, Lee SH, Chae KR, Lim HJ, Min SH, Seo SJ, Song YS, Song CW, Paik SG, Sheen YY, Kim YK (2004) Aberrant expressions of pathogenic phenotype in Alzheimer’s diseased Tg mice carrying NSE-contolled APPsw. Exp Neurol 186:20–32
Lim HJ, Cho JS, Oh JH, Shim SB, Hwang DY, Jee SW, Lee SH, Sheen YY, Lee SH, Kim YK (2005) NSE-controlled carboxyl-terminus of APP gene overexpressing in Tg mice induces altered expressions in behavior, Aβ-42, and GSK3β-binding proteins. Cell Mol Neurobiol 25:833–850
Seo SJ, Hwang DY, Cho JS, Chae KR, Kim CK, Shim SB, Jee SW, Lee SH, Sin JS, Choi SY, Kim J, Kim YK (2007) PEN-2 overexpression induces γ-secretase protein and its activity with amyloid β-42 production. Neurochem Res 32:1016–1023
Takasugi N, Tomita T, Hayashi I, Tsuroka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T (2003) The role of presenilin cofactors in the gamma-secretase complex. Nature 422:385–387
Nguyen V, Hawkins C, Bergeron C, Supala A, Huang J, Westaway D, St George-Hyslop P, Rozmahel R (2006) Loss of nicastrin elicits an apoptotic phenotype in mouse embryos. Brain Res 1086:76–84
Subramaniam D, Ponnurangam S, Ramamoorthy P, Standing D, Battafarano RJ, Anant S, Sharma P (2012) Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PLoS One 7:e30590
Forss-Petter S, Danielson PE, Catsicas S, Battenberg E, Price J, Nerenberg M, Sutcliffe JG (1990) Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron 5:187–197
Kimberly WT, LaVoie MJ, Ostazewski BL, Ye W, Wolfe MS, Selkoe DJ (2003) Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci USA 100:6382–6387
Kim SH, Ikeuchi T, Yu C, Sisodia SS (2003) Regulated hyperaccumulation of presenilin-1 and the “γ-secretase” complex. Evidence for differential intramembranous processing of transmembrane substrates. J Biol Chem 278:33992–34002
Kaether C, Haass C, Steiner H (2006) Assembly, trafficking and function of gamma-secretase. Neurodegener Dis 3:275–283
Shirotani K, Edbauer D, Kostka M, Steiner H, Haasse C (2004) Immature nicastrin stabilizes APH-1 independent of PEN-2 and presenilin: identification of nicastrin mutant that selectively interact with APH-1. J Neurochem 89:1520–1527
Näslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD (2000) Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 283:1571–1577
McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Bayreuther K, Bush AI, Master CL (1999) Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Annul Neurol 46:860–866
Leu LF, Kuo YM, Roher AE, Brachova L, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J (1999) Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol 155:853–862
Pamrén A, Wanngren J, Tjernberg LO, Winblad B, Bhat R, Näslund J, Karlström H (2011) Mutations in nicastrin protein differentially affect amyloid beta-peptide production and Notch protein processing. J Biol Chem 286:31153–31158
Acknowledgments
We thank Sun M. Choi, B.S. and Mi K. Jang, M.S., animal technicians, for directing the animal facility at the Division of Laboratory Animal Resources.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jun Seo Goo and Yong Bin Kim these authors equally contributed to this work.
Rights and permissions
About this article
Cite this article
Goo, J.S., Kim, Y.B., Shim, S.B. et al. Nicastrin Overexpression in Transgenic Mice Induces Aberrant Behavior and APP Processing. Mol Neurobiol 48, 232–243 (2013). https://doi.org/10.1007/s12035-013-8453-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8453-3