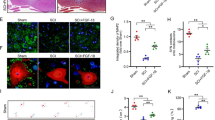
Abstract
The role of autophagy in the recovery of spinal cord injury remains controversial; in particular, the mechanism of autophagy regulated degradation of ubiquitinated proteins has not been discussed to date. In this study, we investigated the protective role of basic fibroblast growth factor (bFGF) both in vivo and in vitro and demonstrated that excessive autophagy and ubiquitinated protein accumulation is involved in the rat model of trauma. bFGF administration improved recovery and increased the survival of neurons in spinal cord lesions in the rat model. The protective effect of bFGF is related to the inhibition of autophagic protein LC3II levels; bFGF treatment also enhances clearance of ubiquitinated proteins by p62, which also increases the survival of neuronal PC-12 cells. The activation of the downstream signals of the PI3K/Akt/mTOR pathway by bFGF treatment was detected both in vivo and in vitro. Combination therapy including the autophagy activator rapamycin partially abolished the protective effect of bFGF. The present study illustrates that the role of bFGF in SCI recovery is related to the inhibition of excessive autophagy and enhancement of ubiquitinated protein clearance via the activation of PI3K/Akt/mTOR signaling. Overall, our study suggests a new trend for bFGF drug development for central nervous system injuries and sheds light on protein signaling involved in bFGF action.








Similar content being viewed by others
Abbreviations
- bFGF:
-
Basic fibroblast growth factor
- SCI:
-
Spinal cord injury
- LC3:
-
Light chain 3
- mTOR:
-
Mammalian target rapamycin
- 3-MA:
-
3-Methyladenine
- BBB:
-
Basso Beattie, and Bresnahan
- UPS:
-
Ubiquitin–proteasome system
- IGF-1:
-
Insulin-like growth factor-1
- NGF:
-
Nerve growth factor
- BDNF:
-
Brain-derived neurotrophic factor
- GDNF:
-
Glial cell line-derived neurotrophic factor
References
Penas C, Guzman MS, Verdu E, Fores J, Navarro X, Casas C (2007) Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J Neurochem 102:1242–1255
Chen HC, Fong TH, Lee AW, Chiu WT (2012) Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine (Phila Pa 1976) 7:470–475
Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E (2011) Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine (Phila Pa 1976) 36:E1427–1434
Katayama M, Kawaguchi T, Berger MS, Pieper RO (2007) DNA damaging agent-induced autophagy produces a cytoprotective adenosine triphosphate surge in malignant glioma cells. Cell Death Differ 14:548–558
Criollo A, Maiuri MC, Tasdemir E, Vitale I, Fiebig AA, Andrews D, Molgo J, Diaz J, Lavandero S, Harper F, Pierron G, di Stefano D, Rizzuto R, Szabadkai G, Kroemer G (2007) Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ 14:1029–1039
Sekiguchi A, Kanno H, Ozawa H, Yamaya S, Itoi E (2012) Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J Neurotrauma 29:946–956
Walker CL, Walker MJ, Liu NK, Risberg EC, Gao X, Chen J, Xu XM (2012) Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One 7:e30012
Chen PC, Bhattacharyya BJ, Hanna J, Minkel H, Wilson JA, Finley D, Miller RJ, Wilson SM (2011) Ubiquitin homeostasis is critical for synaptic development and function. J Neurosci 31:17505–17513
Sanchez P, De Carcer G, Sandoval IV, Moscat J, Diaz-Meco MT (1998) Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction with p62. Mol Cell Biol 18:3069–3080
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P (2009) Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol 41:1817–1827
Kirkin V, Lamark T, Johansen T, Dikic I (2009) NBR1 cooperates with p62 in selective autophagy of ubiquitinated targets. Autophagy 5:732–733
Komatsu M, Ichimura Y (2010) Physiological significance of selective degradation of p62 by autophagy. FEBS Lett 584:1374–1378
Beenken A, Mohammadi M (2009) The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 8:235–253
Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG (2010) Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci 30:1657–1676
Liu WG, Wang ZY, Huang ZS (2011) Bone marrow-derived mesenchymal stem cells expressing the bFGF transgene promote axon regeneration and functional recovery after spinal cord injury in rats. Neurol Res 33:686–693
Lin X, Zhang Y, Liu L, McKeehan WL, Shen Y, Song S, Wang F (2011) FRS2alpha is essential for the fibroblast growth factor to regulate the mTOR pathway and autophagy in mouse embryonic fibroblasts. Int J Biol Sci 7:1114–1121
Zhang J, Liu J, Liu L, McKeehan WL, Wang F (2012) The fibroblast growth factor signaling axis controls cardiac stem cell differentiation through regulating autophagy. Autophagy 8:690–691
Perrin FE, Boniface G, Serguera C, Lonjon N, Serre A, Prieto M, Mallet J, Privat A (2010) Grafted human embryonic progenitors expressing neurogenin-2 stimulate axonal sprouting and improve motor recovery after severe spinal cord injury. PLoS One 5:e15914
Harada M, Hanada S, Toivola DM, Ghori N, Omary MB (2008) Autophagy activation by rapamycin eliminates mouse Mallory–Denk bodies and blocks their proteasome inhibitor-mediated formation. Hepatology 47:2026–2035
Carloni S, Buonocore G, Longini M, Proietti F, Balduini W (2012) Inhibition of rapamycin-induced autophagy causes necrotic cell death associated with Bax/Bad mitochondrial translocation. Neuroscience 203:160–169
Cevikbas F, Steinhoff M, Ikoma A (2011) Role of spinal neurotransmitter receptors in itch: new insights into therapies and drug development. CNS Neurosci Ther 17:742–749
Moon YJ, Lee JY, Oh MS, Pak YK, Park KS, Oh TH, Yune TY (2012) Inhibition of inflammation and oxidative stress by Angelica dahuricae radix extract decreases apoptotic cell death and improves functional recovery after spinal cord injury. J Neurosci Res 90:243–256
Angelucci F, Aloe L, Iannitelli A, Gruber SH, Mathe AA (2005) Effect of chronic olanzapine treatment on nerve growth factor and brain-derived neurotrophic factor in the rat brain. Eur Neuropsychopharmacol 15:311–317
Tanida I (2011) Autophagosome formation and molecular mechanism of autophagy. Antioxid Redox Signal 14:2201–2214
Rubinsztein DC, Codogno P, Levine B (2012) Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 11:709–730
Liberski PP, Gajdusek DC, Brown P (2002) How do neurons degenerate in prion diseases or transmissible spongiform encephalopathies (TSEs): neuronal autophagy revisited. Acta Neurobiol Exp (Wars) 62:141–147
Yousefi S, Simon HU (2007) Apoptosis regulation by autophagy gene 5. Crit Rev Oncol Hematol 63:241–244
Cuervo AM (2004) Autophagy: in sickness and in health. Trends Cell Biol 14:70–77
do Carmo Cunha J, de Freitas Azevedo Levy B, de Luca BA, de Andrade MS, Gomide VC, Chadi G (2007) Responses of reactive astrocytes containing S100beta protein and fibroblast growth factor-2 in the border and in the adjacent preserved tissue after a contusion injury of the spinal cord in rats: implications for wound repair and neuroregeneration. Wound Repair Regen 15:134–146
Nguyen TL, Kim CK, Cho JH, Lee KH, Ahn JY (2010) Neuroprotection signaling pathway of nerve growth factor and brain-derived neurotrophic factor against staurosporine induced apoptosis in hippocampal H19-7/IGF-IR [corrected]. Exp Mol Med 42:583–595
Zhang HY, Zhang X, Wang ZG, Shi HX, Wu FZ, Lin BB, Xu XL, Wang XJ, Fu XB, Li ZY, Shen CJ, Li XK, Xiao J (2013) Exogenous basic fibroblast growth factor inhibits ER stress-induced apoptosis and improves recovery from spinal cord injury. CNS Neurosci Ther 19:20–29
Gammoh N, Lam D, Puente C, Ganley I, Marks PA, Jiang X (2012) Role of autophagy in histone deacetylase inhibitor-induced apoptotic and nonapoptotic cell death. Proc Natl Acad Sci U S A 109:6561–6565
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35
Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY (2012) Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy 8:77–87
Chen L, Xu B, Liu L, Luo Y, Zhou H, Chen W, Shen T, Han X, Kontos CD, Huang S (2011) Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic Biol Med 50:624–632
Castedo M, Ferri KF, Kroemer G (2002) Mammalian target of rapamycin (mTOR): pro- and anti-apoptotic. Cell Death Differ 9:99–100
Chen S, Ferrone FA, Wetzel R (2002) Huntington's disease age-of-onset linked to polyglutamine aggregation nucleation. Proc Natl Acad Sci U S A 99:11884–11889
Dohm CP, Kermer P, Bahr M (2008) Aggregopathy in neurodegenerative diseases: mechanisms and therapeutic implication. Neurodegener Dis 5:321–338
Thorpe JR, Tang H, Atherton J, Cairns NJ (2008) Fine structural analysis of the neuronal inclusions of frontotemporal lobar degeneration with TDP-43 proteinopathy. J Neural Transm 115:1661–1671
Cheng B, Maffi SK, Martinez AA, Acosta YP, Morales LD, Roberts JL (2011) Insulin-like growth factor-I mediates neuroprotection in proteasome inhibition-induced cytotoxicity in SH-SY5Y cells. Mol Cell Neurosci 47:181–190
Pan T, Kondo S, Zhu W, Xie W, Jankovic J, Le W (2008) Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiol Dis 32:16–25
Casas S, Gomis R, Gribble FM, Altirriba J, Knuutila S, Novials A (2007) Impairment of the ubiquitin–proteasome pathway is a downstream endoplasmic reticulum stress response induced by extracellular human islet amyloid polypeptide and contributes to pancreatic beta-cell apoptosis. Diabetes 56:2284–2294
Acknowledgments
This work was supported by Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (to J.X.), National Natural Science Funding of China (81200958, 81200010), State Key Basic Research Development Program (2012CB518105), Zhejiang Provincial Project of Key Group (2010R5004202), Ninbo Natural Science Foundation (2012A610255).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
H.-Y. Zhang Z.-G. Wang and F.-Z. Wu contributed equally to this study.
Electronic supplementary material
Fig. S1
(A) Statistical results of NE staining of sham, SCI model group and SCI model mice treated by bFGF group of Figure 1D. (B) Statistical results of Immunofluorecence staining results of LC3 of Figure 2A. (C) Statistical results of Immunofluorecence staining results of Ub of Figure 4C. (D) Statistical results of Immunofluorecence staining results of LC3 in PC12 cell of Figure 7B. (JPEG 69 kb)
High Resolution Image
(TIFF 128 kb)
Rights and permissions
About this article
Cite this article
Zhang, HY., Wang, ZG., Wu, FZ. et al. Regulation of Autophagy and Ubiquitinated Protein Accumulation by bFGF Promotes Functional Recovery and Neural Protection in a Rat Model of Spinal Cord Injury. Mol Neurobiol 48, 452–464 (2013). https://doi.org/10.1007/s12035-013-8432-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8432-8