Abstract
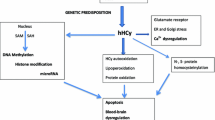
Homocysteine (Hcy) is a thiol-containing amino acid formed during methionine metabolism. Elevated level of Hcy is known as hyperhomocysteinemia (HHcy). HHcy is an independent risk factor for cerebrovascular diseases such as stroke, dementia, Alzheimer’s disease, etc. Stroke, which is caused by interruption of blood supply to the brain, is one of the leading causes of death and disability in a number of people worldwide. The HHcy causes an increased carotid artery plaque that may lead to ischemic stroke but the mechanism is currently not well understood. Though mutations or polymorphisms in the key genes of Hcy metabolism pathway have been well elucidated in stroke, emerging evidences suggested epigenetic mechanisms equally play an important role in stroke development such as DNA methylation, chromatin remodeling, RNA editing, noncoding RNAs (ncRNAs), and microRNAs (miRNAs). However, there is no review available yet that describes the role of genetics and epigenetics during HHcy in stroke. The current review highlights the role of genetics and epigenetics in stroke during HHcy and the role of epigenetics in its therapeutics. The review also highlights possible epigenetic mechanisms, potential therapeutic molecules, putative challenges, and approaches to deal with stroke during HHcy.



Similar content being viewed by others
References
Mathers CD, Boerma T, Ma Fat D (2009) Global and regional causes of death. Br Med Bull 92:7–32
Donnan GA, Fisher M, Macleod M, Davis SM (2008) Stroke. Lancet 371:1612–1623
Hankey GJ, Eikelboom JW (2005) Homocysteine and stroke. Lancet 365:194–196
Harker LA, Ross R, Slichter SJ, Scott CR (1976) Homocysteine induced arteriosclerosis: the role of endothelial cell injury and platelet response in its genesis. J Clin Invest 58:731–741
Nishinaga M, Ozawa T, Shimada K (1993) Homocysteine, a thrombogenic agent, suppresses anticoagulant heparin sulfate expression in cultured porcine aortic endothelial cells. J Clin Invest 92:1381–1386
Ratnoff OD (1968) Activation of Hageman factor by L-homocystine. Science 162:1007–1009
Wald DS, Law M, Morris JK (2002) Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. Br Med J 325:1202–1206
McIlroy SP, Dynan KB, Lawson JT, Patterson CC, Passmore AP (2002) Moderately high homocysteine tied to stroke, Alzheimer’s risk. Science news Oct 4
Biswas A, Ranjan R, Meena A, Akhter MS, Yadav BK et al (2009) Homocystine levels, polymorphisms and the risk of ischemic stroke in young Asian Indians. J Stroke Cerebrovasc Dis 18:103–110
Casas JP, Bautista LE, Smeeth L, Sharma P, Hingorani AD (2005) Homocysteine and stroke: evidence on a causal link from Mendelian randomisation. Lancet 365:224–232
Fang L, Wu W, Wu YQ (2004) Relationship between polymorphisms of cystathionine beta-synthase gene and stroke. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 16:161–164
Jeyaseelan K, Lim KY, Armugam A (2008) MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 39:959–966
Thambyrajah J, Townend JN (2000) Homocysteine and atherothrombosis: mechanisms for injury. Eur Heart J 21:967–974
Low H-Q, Chen Christopher PLH, Kasiman K, Thalamathu A, Ng S-S et al (2011) A comprehensive association analysis of homocysteine metabolic pathway genes in Singaporean Chinese with ischemic stroke. PLoS One 6:24757
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA et al (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10:111–113
Robertson KD (2005) DNA methylation and human disease. Nat Rev Genet 6(597):610
Souto JC, Blanco-Vaca F, Soria JM et al (2005) A genome wide exploration suggests a new candidate gene at chromosome 11q23 as the major determinant of plasma homocysteine levels: results from the GAIT project. Am J Hum Genet 76:925–933
Hu CJ, Chen SD, Yang DI et al (2006) Promoter region methylation and reduced expression of thrombospondin-1 after oxygen-glucose deprivation in murine cerebral endothelial cells. J Cereb Blood Flow Metab 26:1519–1526
Zhou HJ, Zhang HN, Tang T et al (2010) Alteration of thrombospondin-1 and -2 in rat brains following experimental intracerebral hemorrhage. J Neurosurg 113:820–825
Langley B, Brochier C, Rivieccio MA (2009) Targeting histone deacetylases as a multifaceted approach to treat the diverse outcomes of stroke. Stroke 40:2899–2905
Ubbink JB (2000) Assay methods for the measurement of total homocyst(e)ine in plasma. Semin Thromb Hemost 26:233–241
Kang SS, Zhou J, Wong PW, Kowalisyn J, Strokosch G (1988) Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet 43:414–421
Zhang W, Sun K, Chen J, Liao Y, Qin Q et al (2010) High plasma homocysteine levels contribute to the risk of stroke recurrence and all-cause mortality in a large prospective stroke population. Clin Sci 118:187–194
Morita H, Kurihara H, Sugiyama T, Hamada C, Kurihara Y et al (1999) Polymorphism of the methionine synthase gene: association with homocysteine metabolism and late-onset vascular diseases in the Japanese population. Arterioscler Thromb Vasc Biol 19:298–302
Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH (1993) Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA 270:2693–2698
Mehler MF (2008) Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Prog Neurobiol 86:305–341
Mehler MF, Mattick JS (2007) Noncoding RNAs and RNA editing in brain development, functional diversification, and neurological disease. Physiol Rev 87:799–823
Qureshi IA, Mehler MF (2010) Emerging role of epigenetics in stroke: part 1: DNA methylation and chromatin modifications. Arch Neurol 67:1316–1322
Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS (2009) Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol 5:401–408
Endres M, Meisel A, Biniszkiewicz D et al (2000) DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci 20:3175–3181
Endres M, Fan G, Meisel A, Dirnagl U, Jaenisch R (2001) Effects of cerebral ischemia in mice lacking DNA methyltransferase 1 in post-mitotic neurons. Neuroreport 12:3763–3766
Sunday L, Osuna C, Krause DN, Duckles SP (2007) Age alters cerebrovascular inflammation and effects of estrogen. Am J Physiol Heart Circ Physiol 292:2333–2340
Wilson ME, Westberry JM (2009) Regulation of oestrogen receptor gene expression: new insights and novel mechanisms. J Neuroendocrinol 21:238–242
Nicolas FE, Pais H, Schwach F et al (2008) Experimental identification of microRNA-140 targets by silencing and overexpressing miR-140. RNA 14:2513–2520
Chen M, Gavrilova O, Liu J (2005) Alternative Gnas gene products have opposite effects on glucose and lipid metabolism. Proc Natl Acad Sci U S A 102:7386–7391
Dobrovolny R, Dvorakova L, Ledvinova J et al (2005) Relationship between X-inactivation and clinical involvement in Fabry heterozygotes: eleven novel mutations in the alphagalactosidase A gene in the Czech and Slovak population. J Mol Med 83:647–654
Freson K, Izzi B, Labarque V et al (2008) GNAS defects identified by stimulatory G protein alpha-subunit signalling studies in platelets. J Clin Endocrinol Metab 93:4851–4859
Giacomini PS, Shannon PT, Clarke JT, Jaigobin C (2004) Fabry’s disease presenting as stroke in a young female. Can J Neurol Sci 31:112–114
Kusuhara T, Ayabe M, Hino H, Shoji H, Neshige R (1996) A case of Prader–Willi syndrome with bilateral middle cerebral artery occlusion and moyamoya phenomenon. Rinsho Shinkeigaku 36:770–773
Cairns BR (2009) The logic of chromatin architecture and remodeling at promoters. Nature 461:193–198
Jenuwein T, Allis CD (2001) Translating the histone code. Science 293(5532):1074–1080
Kouzarides T (2007) Chromatin modifications and their function. Cell 128:693–705
Fang S, Miao J, Xiang L, Ponugoti B, Treuter E, Kemper JK (2007) Coordinated recruitment of histone methyltransferase G9a and other chromatin-modifying enzymes in SHP-mediated regulation of hepatic bile acid metabolism. Mol Cell Biol 27:1407–1424
Gilardi F, Mitro N, Godio C et al (2007) The pharmacological exploitation of cholesterol 7 alpha-hydroxylase, the key enzyme in bile acid synthesis: from binding resins to chromatin remodelling to reduce plasma cholesterol. Pharmacol Ther 116:449–472
Shafaati M, O'Driscoll R, Björkhem I, Meaney S (2009) Transcriptional regulation of cholesterol 24-hydroxylase by histone deacetylase inhibitors. Biochem Biophys Res Commun 378:689–694
Finkel T, Deng CX, Mostoslavsky R (2009) Recent progress in the biology and physiology of sirtuins. Nature 460:587–591
Faraco G, Pancani T, Formentini L et al (2006) Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol 70:1876–1884
Soriano FX, Papadia S, Bell KF, Hardingham GE (2009) Role of histone acetylation in the activity-dependent regulation of sulfiredoxin and sestrin 2. Epigenetics 4:152–158
Birney E, Stamatoyannopoulos JA, Dutta A et al (2007) Identification and analysis of functional elements in 1 % of the human genome by the ENCODE pilot project. Nature 447:799–816
Mattick JS, Amaral PP, Dinger ME, Mercer TR, Mehler MF (2009) RNA regulation of epigenetic processes. Bioessays 31:51–59
Schratt G (2009) Fine-tuning neural gene expression with microRNAs. Curr Opin Neurobiol 19:213–219
Dharap A, Bowen K, Place R, Li LC, Vemuganti R (2009) Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 29:675–687
Mercer TR, Dinger ME, Mattick JS (2009) Long non-coding RNAs: insights into functions. Nat Rev Genet 10:155–159
Rashidian J, Iyirhiaro G, Aleyasin H et al (2005) Multiple cyclin-dependent kinases signals are critical mediators of ischemia/hypoxic neuronal death in vitro and in vivo. Proc Natl Acad Sci U S A 102:14080–14085
Wang X, Arai S, Song X et al (2008) Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature 454:126–130
Broadbent HM, Peden JF, Lorkowski S et al (2008) Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum Mol Genet 17:806–814
Holdt LM, Beutner F, Scholz M et al (2010) ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler Thromb Vasc Biol 30:620–627
Jarinova O, Stewart AF, Roberts R et al (2009) Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arterioscler Thromb Vasc Biol 29:1671–1677
Liu Y, Sanoff HK, Cho H et al (2009) INK4/ARF transcript expression is associated with chromosome 9p21 variants linked to atherosclerosis. PLoS One 4:5027
Pasmant E, Laurendeau I, He'ron D, Vidaud M, Vidaud D, Bièche I (2007) Characterization of a germ-line deletion, including the entire INK4/ARF locus, in amelano-maneural system tumor family. Cancer Res 67:3963–3969
Schaefer AS, Richter GM, Groessner-Schreiber B et al (2009) Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS Genet 5:1000378
Visel A, Zhu Y, May D et al (2010) Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature 464:409–412
Costain WJ, Rasquinha I, Graber T et al (2006) Cerebral ischemia induces neuronal expression of novel VL30 mouse retrotransposons bound to polyribosomes. Brain Res 1094:24–37
Kawahara Y, Megraw M, Kreider E et al (2008) Frequency and fate of microRNA editing in human brain. Nucleic Acids Res 36:5270–5280
Yang W, Chendrimada TP, Wang Q et al (2006) Modulation of microRNA processing and expression through RNA editing by ADAR deaminases. Nat Struct Mol Biol 13:13–21
Kawahara Y, Zinshteyn B, Chendrimada TP, Shiekhattar R, Nishikura K (2007) RNA editing of the microRNA-151 precursor blocks cleavage by the Dicer–TRBP complex. EMBO Rep 8:763–769
Kim JB, Piao CS, Lee KW et al (2004) Delayed genomic responses to transient middle cerebral artery occlusion in the rat. J Neurochem 89:1271–1282
Shani V, Bromberg Y, Sperling O, Zoref-Shani E (2009) Involvement of Src tyrosine kinases (SFKs) and of focal adhesion kinase (FAK) in the injurious mechanism in rat primary neuronal cultures exposed to chemical ischemia. J Mol Neurosci 37:50–59
Shimamura N, Matchett G, Yatsushige H, Calvert JW, Ohkuma H, Zhang J (2006) Inhibition of integrin αvβ3 ameliorates focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Stroke 37:1902–1909
Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS (2003) Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol 183:355–366
Lee TH, Yoon JG (2008) Intracerebral transplantation of human adipose tissue stromal cells after middle cerebral artery occlusion in rats. J Clin Neurosci 15:907–912
Plummer R, Vidal L, Griffin M et al (2009) Phase I study of MG98, an oligonucleotide antisense inhibitor of human DNA methyltransferase 1, given as a 7-day infusion in patients with advanced solid tumors. Clin Cancer Res 5:3177–3183
Csoka AB, Szyf M (2009) Epigenetic side-effects of common pharmaceuticals: a potential new field in medicine and pharmacology. Med Hypotheses 73:770–780
Milutinovic S, D'Alessio AC, Detich N, Szyf M (2007) Valproate induces widespread epigenetic reprogramming which involves demethylation of specific genes. Carcinogenesis 28:560–571
Phiel CJ, Zhang F, Huang EY et al (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276:36734–36741
Szyf M (2010) Epigenetic therapeutics in autoimmune disease. Clin Rev Allergy Immunol 39:62–77
Lu KT, Robin YY, Chen LG et al (2006) Neuroprotective effects of resveratrol on cerebral ischemia-induced neuron loss mediated by free radical scavenging and cerebral blood flow elevation. J Agric Food Chem 54:3126–3131
Ovbiagele B (2008) Potential role of curcumin in stroke prevention. Expert Rev Neurother 8:1175–1176
Zhang Z, Yang X, Zhang S, Ma X, Kong J (2007) BNIP3 upregulation and EndoG translocation in delayed neuronal death in stroke and in hypoxia. Stroke 38:1606–1613
Miyawaki T, Ofengeim D, Noh KM et al (2009) The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci 12:618–626
Qureshi IA, Mehler MF (2009) Regulation of non-coding RNA networks in the nervous system: what’s the REST of the story? Neurosci Lett 466:73–80
Calderone A, Jover T, Noh KM et al (2003) Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci 23:2112–2121
Formisano L, Noh KM, Miyawaki T, Mashiko T, Bennett MV, Zukin RS (2007) Ischemic insults promote epigenetic reprogramming of μ opioid receptor expression in hippocampal neurons. Proc Natl Acad Sci U S A 104:4170–4175
Li JB, Levanon EY, Yoon JK et al (2009) Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science 324:1210–1213
Feinberg EH, Hunter CP (2003) Transport of dsRNA into cells by the transmembrane protein SID-1. Science 301:1545–1547
Sproule DM, Kaufmann P (2008) Mitochondrial encephalopathy, lactic acidosis, and stroke like episodes. Ann N Y Acad Sci 1142:133–158
Smalheiser NR (2007) Exosomal transfer of proteins and RNAs at synapses in the nervous system. Biol Direct 2:35
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345
Mizrak A, Bolukbasi MF, Ozdener GB et al (2013) Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther 21:101–108
Acknowledgments
This work was supported by NIH grant HL-107640 to NT and NS-51568 to SCT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalani, A., Kamat, P.K., Tyagi, S.C. et al. Synergy of Homocysteine, MicroRNA, and Epigenetics: A Novel Therapeutic Approach for Stroke. Mol Neurobiol 48, 157–168 (2013). https://doi.org/10.1007/s12035-013-8421-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8421-y