Abstract
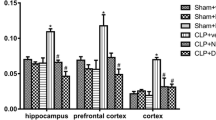
In the present study, we investigated whether sepsis induced by cecal ligation and puncture (CLP) modifies Na+, K+-ATPase activity, mRNA expression, and cerebral edema in hippocampus and cerebral cortex of rats and if antioxidant (ATX) treatment prevented the alterations induced by sepsis. Rats were subjected to CLP and were divided into three groups: sham; CLP—rats were subjected to CLP without any further treatment; and ATX–CLP plus administration of N-acetylcysteine plus deferoxamine. Several times (6, 12, and 24) after CLP or sham operation, the rats were killed and hippocampus and cerebral cortex were isolated. Na+, K+-ATPase activity was inhibited in the hippocampus 24 h after sepsis, and ATX treatment was not able to prevent this inhibition. The Na+, K+-ATPase activity also was inhibited in cerebral cortex 6, 12, and 24 h after sepsis. No differences on Na+, K+-ATPase catalytic subunit mRNA levels were found in the hippocampus and cerebral cortex after sepsis. ATX treatment prevents Na+, K+-ATPase inhibition only in the cerebral cortex. Na+, K+-ATPase inhibition was not associated to increase brain water content. In conclusion, the present study demonstrated that sepsis induced by CLP inhibits Na+, K+-ATPase activity in a mechanism dependent on oxidative stress, but this is not associated to increase brain water content.




Similar content being viewed by others
References
Vandijck D, Decruyenaere JM, Blot SI (2006) The value of sepsis definitions in daily ICU-practice. Acta Clin Belg 6:220–226
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–142
Wheeler AP, Bernard GR (1999) Treating patients with severe sepsis. N Engl J Med 340:207–214
Salvemini D, Cuzzocrea S (2002) Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic Biol Med 33:1173–1185
Barichello T, Fortunato JJ, Vitali AM, Feier G, Reinke A, Moreira JC, Quevedo J, Dal-Pizzol F (2006) Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med 34:886–889
Andrades ME, Ritter C, Dal-Pizzol F (1994) The role of free radicals in sepsis development. Front Biosci 1:277
Ericinska M, Silver IA (1994) Silver, ions and energy in mammalian brain. Prog Neurobiol 16:37–71
Lees GJ (1991) Inhibition of sodium-potassium-ATPase: a potentially ubiquitous mechanism contributing to central nervous system neuropathology. Brain Res Rev 16:283–300
Wyse AT, Streck EL, Barros SV, Brusque AM, Zugno AI, Wajner M (2000) Methylmalonate administration decreases Na+, K+-ATPase activity in cerebral cortex of rats. Neuroreport 11:2331–2334
Wyse ATS, Streck EL, Worm P, Wajner A, Ritter F, Netto CA (2000) Preconditioning prevents the inhibition of Na+, K+-ATPase activity after brain ischemia. Neurochem Res 25:969–973
Yu SP (2003) Na+, K+-ATPase: the new face of an old player in pathogenesis and apoptotic/hybrid cell death. Biochem Pharmacol 66:1601–1609
Hernandez JR (1992) Na+, K+-ATPase regulation by serotonin in normal and kindled rats. Brain Res 593:239–244
Yang ZJ, Torbey M, Li X, Bernardy J, Golden WC, Martin LJ, Koehler RC (2007) Dopamine receptor modulation of hypoxicischemic neuronal injury in striatum of newborn piglets. J Cereb Blood Flow Metab 27:1339–1351
Kurella E, Kukley M, Tyulina O, Dobrota D, Matejovicova M, Mezesova V, Boldyrev A (1997) Kinetic parameters of Na+/K+-ATPase modified by free radicals in vitro and in vivo. Ann N Y Acad Sci 834:661–665
Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JC, Dal-Pizzol F (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349
Ritter C, Andrades ME, Frota MLC, Bonatto F, Pinho RA, Polydoro M, Klamt F, Pinheiro CT, Menna-Barreto SS, Moreira JC, Dal-Pizzol F (2003) Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intensive Care Med 29:1782–1789
Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal-Pizzol F (2005) Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med 33:221–223
Bradford MM (1976) A rapid and sensitive method for the quantification of micrograms quantities of protein utilizing the principle of protein-die-binding. Anal Biochem 72:248–254
Chan KM, Delfer D, Junger KD (1986) A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem 157:375–380
Durmaz R, Ertilav K, Akyuz F, Kanbak G, Bildirici K, Tel E (2003) Lazaroid U-74389G attenuates edema in rat brain subjected to post-ischemic reperfusion injury. J Neurol Sci 215:87–93
Zhang H, Slutsky AS, Vincent JL (2000) Oxygen free radicals in ARDS, septic shock and organ dysfunction. Intensive Care Med 26:474–476
Zapelini PH, Rezin GT, Cardoso MR, Ritter C, Klamt F, Moreira JC, Streck EL, Dal-Pizzol F (2008) Antioxidant treatment reverses mitochondrial dysfunction in a sepsis animal model. Mitochondrion 8:211–218
Joshi MS, Julian MW, Huff JE, Bauer JA, Xia Y, Crouser ED (2006) Calcineurin regulates myocardial function during acute endotoxemia. Am J Respir Crit Care Med 173:999–1007
Protti A, Carre J, Frost MT, Taylor V, Stidwill R, Rudiger A, Singer M (2007) Succinate recovers mitochondrial oxygen consumption in septic rat skeletal muscle. Crit Care Med 35:2150–2155
Comim CM, Rezin GT, Scaini G, Di-Pietro PB, Cardoso MR, Petronilho FC, Ritter C, Streck EL, Quevedo J, Dal-Pizzol F (2008) Mitochondrial respiratory chain and creatine kinase activities in rat brain after sepsis induced by cecal ligation and perforation. Mitochondrion 8:313–318
Cassol OJ Jr, Rezin GT, Petronilho FC, Scaini G, Gonçalves CL, Ferreira GK, Roesler R, Schwartsmann G, Dal-Pizzol F, Streck EL (2010) Effects of N-acetylcysteine/deferoxamine, taurine and RC-3095 on respiratory chain complexes and creatine kinase activities in rat brain after sepsis. Neurochem Res 35:515–521
Barichello T, Machado RA, Constantino L, Valvassori SS, Réus GZ, Martins MR, Petronilho F, Ritter C, Quevedo J, Dal-Pizzol F (2007) Antioxidant treatment prevented late memory impairment in an animal model of sepsis. Crit Care Med 35:2186–2190
Sweadner KJ (1979) Two molecular forms of Na(+) K(+)-stimulated ATPase in brain. Separation, and difference in affinity for strophanthidin. J Biol Chem 254:6060–6067
Papadopoulos MC, Lamb FJ, Moss RF, Davies DC, Tighe D, Bennett ED (1999) Fecal peritonitis causes edema and neuronal injury in pig cerebral cortex. Clin Sci (Colch) 96:461–466
Comim CM, Vilela MC, Constantino LS, Petronilho F, Vuolo F, Lacerda-Queiroz N, Rodrigues DH, da Rocha JL, Teixeira AL, Quevedo J, Dal-Pizzol F (2011) Traffic of leukocytes and cytokine up-regulation in the central nervous system in sepsis. Intensive Care Med 37:711–718
Wang XQ, Xiao AY, Sheline C, Hyrc K, Yang A, Goldberg MP, Choi DW, Yu SP (2003) Apoptotic insults impair Na+, K+-ATPase activity as a mechanism of neuronal death mediated by concurrent ATP deficiency and oxidant stress. J Cell Sci 116:2099–2110
Beguin P, Beggah AT, Chibalin AV, Burgener-Kairuz P, Jaisser F, Mathews PM, Rossier BC, Cotecchia S, Geering K (1994) Phosphorylation of the Na, K-ATPase alpha-subunit by protein kinase A and C in vitro and in intact cells. Identification of a novel motif for PKC-mediated phosphorylation. J Biol Chem 269:24437–24445
Feschenko MS, Sweadner KJS (1995) Structural basis for species-specific differences in the phosphorylation of Na, K-ATPase by protein kinase C. J Biol Chem 270:14072–14077
Robinson JD, Pratap PR (1993) Indicators of conformational changes in the Na+/K(+)-ATPase and their interpretation. Biochim Biophys Acta 1154:83–104
Logvinenko NS, Dulubova I, Fedosova N, Larsson SH, Nairn AC, Esmann M, Greengard P, Aperia A (1996) Phosphorylation by protein kinase C of serine-23 of the α-1 subunit of rat Na+, K+-ATPase affects its conformational equilibrium. Proc Natl Acad Sci USA 93:9132–9137
Mishra OP, Delivoria-Papadopoulos M, Cahillane G, Wagerle LC (1989) Lipid peroxidation as the mechanism of modification of the affinity of the Na+, K+-ATPase active sites for ATP, K1, Na1, and strophanthidin in vitro. Neurochem Res 14:845–851
Viani P, Cervato G, Fiorilli A, Cestaro B (1991) Agerelated differences in synaptosomal peroxidative damage and membrane properties. J Neurochem 56:253–258
Yufu K, Itoh T, Edamatsu R, Mori A, Hirakawa M (1993) Effect of hyperbaric oxygenation on the Na+, K+-ATPase and membrane fluidity of cerebrocortical membranes after experimental subarachnoid hemorrhage. Neurochem Res 16:1033–1039
Perl M, Chung CS, Swan R, Ayala A (2007) Role of programmed cell death in the immunopathogenesis of sepsis. Drug Discov Today Dis Mech 4:223–230
Ayala A, Perl M, Venet F, Lomas-Neira J, Swan R, Chung CS (2008) Apoptosis in sepsis: mechanisms, clinical impact and potential therapeutic targets. Curr Pharm Des 14:1853–1859
Messaris E, Memos N, Chatzigianni E, Konstadoulakis MM, Menenakos E, Katsaragakis S, Voumvourakis C, Androulakis G (2004) Time-dependent mitochondrial mediated programmed neuronal cell death prolongs survival in sepsis. Crit Care Med 32:1764–1770
Sharshar T, Annane D, de laGrandmaison GF, Brouland JP, Hopkinson NS, Gray F (2004) The neuropathology of septic shock. Brain Pathol 14:21–33
Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT (2005) Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neu 30:144–157
Heyland DK, Hopman W, Coo H, Tranmer J, McColl MA (2000) Long-term health-related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health-related quality of life. Crit Care Med 28:3599–3605
Acknowledgments
This research was supported by grants from Programa de Pós-graduação em Ciências da Saúde—UNESC and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Author information
Authors and Affiliations
Corresponding author
Additional information
Isabela Casagrande Jeremias and Giselli Scaini contribute equally to this work.
Rights and permissions
About this article
Cite this article
Jeremias, I.C., Scaini, G., Constantino, L. et al. The Decrease on Na+, K+-ATPase Activity in the Cortex, but not in Hippocampus, is Reverted by Antioxidants in an Animal Model of Sepsis. Mol Neurobiol 46, 467–474 (2012). https://doi.org/10.1007/s12035-012-8297-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8297-2