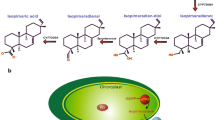
Abstract
Podophyllotoxin (PPT) is a plant natural product that serves as a precursor for the synthesis of many well-known chemotherapeutic drugs. The limited availability and high demand for the source plants of PPT have led to the exploration of alternative sources for this compound. In this study, we utilized the endophytic fungus Phialocephala podophylli (strain PPE7) that we isolated from the rhizomes of Podophyllum peltatum and is known to produce detectable amounts of PPT in broth culture. To date, the complete PPT biosynthetic pathway has yet to be determined in any species. Since fungi are well known for clustering genes that belong to secondary metabolite pathways, use of a fungal system for investigation of the PPT biosynthesis genes may ultimately lead to elucidation of the entire pathway. In this study, we investigated the secoisolariciresinol dehydrogenase (SD) gene that facilitates the dehydrogenation of secoisolariciresinol to form matairesinol, a mid-pathway intermediate product in PPT biosynthesis. We utilized PCR amplification to acquire the complete SD gene sequence in PPE7 and opted to synthesize the P. peltatum SD sequence for expression. Through western blotting, we confirmed the expression of the recombinant SD (PpSD) and verified protein functionality with a bioconversion assay followed by HPLC and LC–MS analyses. Here, we report the identification of the SD gene in PPE7; this is the first report of the SD gene in an endophytic fungus. Additionally, we established the groundwork for the future expression of the complete PPT biosynthetic pathway in the heterologous host Pichia pastoris.









Similar content being viewed by others
References
Canel, C., Moraes, R. M., Dayan, F. E., & Ferreira, D. (2000). Podophyllotoxin. Phytochemistry, 54, 115–120. doi:10.1016/S0031-9422(00)00094-7.
Hartmann, J. T., & Lipp, H.-P. (2006). Camptothecin and podophyllotoxin derivatives. Drug Safety, 29, 209–230. doi:10.2165/00002018-200629030-00005.
Holthuis, J. (1988). Etoposide and teniposide. Pharmaceutisch Weekblad, 10, 101–116.
Liu, Y.-Q., Tian, J., Qian, K., Zhao, X.-B., Morris-Natschke, S. L., Yang, L., et al. (2015). Recent progress on C-4-modified podophyllotoxin analogs as potent antitumor agents. Medicinal Research Reviews, 35, 1–62. doi:10.1002/med.21319.
Roy, A., Ernsting, M. J., Undzys, E., & Li, S.-D. (2015). A highly tumor-targeted nanoparticle of podophyllotoxin penetrated tumor core and regressed multidrug resistant tumors. Biomaterials, 52, 335–346. doi:10.1016/j.biomaterials.2015.02.041.
Moraes, R. M., Bedir, E., Barrett, H., Burandt, C, Jr, Canel, C., & Khan, I. A. (2002). Evaluation of Podophyllum peltatum accessions for podophyllotoxin production. Planta Medica, 68, 341–344. doi:10.1055/s-2002-26740.
Moraes, R. M., Burandt, C., Ganzera, M., Xingli, L., Khan, I., & Canel, C. (2000). The American mayapple revisited-Podophyllum peltatum-still a potential cash crop? Economic Botany, 54, 471–476.
Rust, R., & Roth, R. (1981). Seed production and seedling establishment in the mayapple, Podophyllum peltatum L. American Midland Naturalist, 105, 51–60.
Xu, H., Lv, M., & Tian, X. (2009). A review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: 2003-2007. Current Medicinal Chemistry, 16, 327–349.
Anbazhagan, V., Ahn, C., Harada, E., Kim, Y., & Choi, Y. (2008). Podophyllotoxin production via cell and adventitious root cultures of Podophyllum peltatum. In Vitro Cell Dev, 44, 494–501. doi:10.1007/s11627-008-9134-1.
Mingoia, F., Vitale, M., Madec, D., Prestat, G., & Poli, G. (2008). Pseudo-domino palladium-catalyzed allylic alkylation/Mizoroki–Heck coupling reaction: A key sequence toward (±)-podophyllotoxin. Tetrahedron Letters, 49, 760–763. doi:10.1016/j.tetlet.2007.11.202.
Ward, R. (1990). Asymmetric synthesis of lignans. Tetrahedron, 46, 5029–5041. doi:10.1016/S0040-4020(01)87810-8.
Wu, Y., Zhang, H., Zhao, Y., Zhao, J., Chen, J., & Li, L. (2007). A new and efficient strategy for the synthesis of podophyllotoxin and its analogues. Organic Letters, 9, 1199–1202. doi:10.1021/ol0630954.
Wu, Y., Zhao, J., Chen, J., Pan, C., Li, L., & Zhang, H. (2008). Enantioselective sequential conjugate addition-allylation reactions: A concise total synthesis of (+)-podophyllotoxin. Organic Letters, 11, 597–600. doi:10.1021/ol8026208.
Eyberger, A. L., Dondapati, R., & Porter, J. R. (2006). Endophyte fungal isolates from Podophyllum peltatum produce podophyllotoxin. Journal of Natural Products, 69, 1121–1124. doi:10.1021/np060174f.
Heinig, U., Scholz, S., & Jennewein, S. (2013). Getting to the bottom of Taxol biosynthesis by fungi. Fungal Diversity, 60, 161–170. doi:10.1007/s13225-013-0228-7.
Keller, N. P., & Hohn, T. M. (1997). Metabolic pathway gene clusters in filamentous fungi. Fungal Genetics and Biology, 21, 17–29. doi:10.1006/fgbi.1997.0970.
Davin, L. B., Wang, H.-B., Crowell, A. L., Bedgar, D. L., Martin, D. M., Sarkanen, S., & Lewis, N. G. (1997). Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science, 275, 362–367. doi:10.1126/science.275.5298.362.
Marques, J. V., Kim, K.-W., Lee, C., Costa, M. A., May, G. D., Crow, J. A., et al. (2013). Next generation sequencing in predicting gene function in podophyllotoxin biosynthesis. Journal of Biological Chemistry, 288, 466–479. doi:10.1074/jbc.M112.400689.
Umezawa, T. (2003). Diversity in lignan biosynthesis. Phytochemistry Reviews, 2, 371–390.
Davin, L. B., & Lewis, N. G. (2000). Dirigent proteins and dirigent sites explain the mystery of specificity of radical precursor coupling in lignan and lignin biosynthesis. Plant Physiology, 123, 453–462. doi:10.1104/pp.123.2.453.
Xia, Z.-Q., Costa, M. A., Pélissier, H. C., Davin, L. B., & Lewis, N. G. (2001). Secoisolariciresinol dehydrogenase purification, cloning, and functional expression implications for human health protection. Journal of Biological Chemistry, 276, 12614–12623. doi:10.1074/jbc.M008622200.
Moinuddin, S. G., Youn, B., Bedgar, D. L., Costa, M. A., Helms, G. L., Kang, C., et al. (2006). Secoisolariciresinol dehydrogenase: Mode of catalysis and stereospecificity of hydride transfer in Podophyllum peltatum. Organic & Biomolecular Chemistry, 4, 808–816. doi:10.1039/B516563F.
Youn, B., Moinuddin, S. G., Davin, L. B., Lewis, N. G., & Kang, C. (2005). Crystal structures of apo-form and binary/ternary complexes of Podophyllum secoisolariciresinol dehydrogenase, an enzyme involved in formation of health-protecting and plant defense lignans. Journal of Biological Chemistry, 280, 12917–12926. doi:10.1074/jbc.M413266200.
Harju, S., Fedosyuk, H., & Peterson, K. R. (2004). Rapid isolation of yeast genomic DNA: Bust n’ Grab. BMC Biotechnology, 4, 8. doi:10.1186/1472-6750-4-8.
Altschul, S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W., & Lipman, D. J. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research, 25, 3389–3402. doi:10.1093/nar/25.17.3389.
Zhang, Y. (2008). I-TASSER server for protein 3D structure prediction. BMC Bioinformatics, 9, 40. doi:10.1186/1471-2105-9-40.
Kushnirov, V. V. (2000). Rapid and reliable protein extraction from yeast. Yeast, 16, 857–860.
Kuo, H.-J., Wei, Z.-Y., Lu, P.-C., Huang, P.-L., & Lee, K.-T. (2014). Bioconversion of pinoresinol into matairesinol by use of recombinant Escherichia coli. Applied and Environment Microbiology, 80, 2687–2692. doi:10.1128/AEM.03397-13.
Holst-Jensen, A., Vaage, M., Schumacher, T., & Johansen, S. (1999). Structural characteristics and possible horizontal transfer of group I introns between closely related plant pathogenic fungi. Molecular Biology and Evolution, 16, 114–126.
Ito, Y., & Hirano, T. (1999). A group I intron in the 18S ribosomal DNA from the parasitic fungus Isaria japonica. Journal of Molecular Evolution, 48, 337–340.
Nishida, H., Tajiri, Y., & Sugiyama, J. (1998). Multiple origins of fungal group I introns located in the same position of nuclear SSU rRNA gene. Journal of Molecular Evolution, 46, 442–448.
Perotto, S., Nepote-Fus, P., Saletta, L., Bandi, C., & Young, J. P. W. (2000). A diverse population of introns in the nuclear ribosomal genes of ericoid mycorrhizal fungi includes elements with sequence similarity to endonuclease-coding genes. Molecular Biology and Evolution, 17, 44–59.
Kazenwadel, C., Klebensberger, J., Richter, S., Pfannstiel, J., Gerken, U., Pickel, B., et al. (2013). Optimized expression of the dirigent protein AtDIR6 in Pichia pastoris and impact of glycosylation on protein structure and function. Applied Microbiology and Biotechnology, 97, 7215–7227. doi:10.1007/s00253-012-4579-x.
Cregg, J., Tschopp, J., Stillman, C., Siegel, R., Akong, M., Craig, W., et al. (1987). High-level expression and efficient assembly of hepatitis B surface antigen in the methylotrophic yeast, Pichia pastoris. Nature Biotechnology, 5, 479–485. doi:10.1038/nbt0587-479.
Acknowledgments
We acknowledge and thank the Department of Biological Sciences and the College of Graduate Studies, University of the Sciences in Philadelphia, for support of this project. We also thank Charles McEwen and Sarah Saylor for assistance in the LC–MS experiments. Early parts of this study were supported, in part, by NIH Grant 1R15CA135589-01A1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arneaud, S.L.B., Porter, J.R. Investigation and Expression of the Secoisolariciresinol Dehydrogenase Gene Involved in Podophyllotoxin Biosynthesis. Mol Biotechnol 57, 961–973 (2015). https://doi.org/10.1007/s12033-015-9888-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-015-9888-8