Abstract
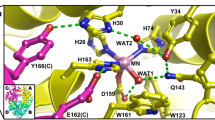
Metal binding of superoxide dismutase from Thermus thermophilus HB27 was analyzed by comparing the related structures and sequences from different origins. Mutants (Ile166Leu, Asp167Glu, and Ile166Leu-Asp167Glu) were prepared and characterized. The mutants Asp167Glu and Ile166Leu-Asp167Glu changed their binding specificities from manganese to iron, which were manifested by the differences in color of the enzyme solutions and by flame atomic absorption analysis. Specific activities of the three mutants were 112, 52, and 62% of that of the wild-type enzyme, respectively. Asp167Glu and Ile166Leu-Asp167Glu only retained 6.8 and 6.1%, respectively, of the original activities after dialysis against 1 mM EDTA. Tryptophan fluorescence measurement and native gel electrophoresis implied that the three mutants could fold into a less condensed structure. Their folding and changes in the ion binding sites of the modeled structures might be the reason for their low affinities to metal ions. These findings increased our understanding of metal binding specificity of superoxide dismutase.






Similar content being viewed by others
References
Amano, A., Shizukuishi, S., Tamagawa, H., Iwakura, K., Tsunasawa, S., & Tsunemitsu, A. (1990). Characterization of superoxide dismutases purified from either anaerobically maintained or aerated Bacteroides gingivalis. Journal of Bacteriology, 172, 1457–1463.
Yamakura, F., Rardin, R. L., Petsko, G. A., Ringe, D., Hiraoka, B. Y., Nakayama, K., et al. (1998). Inactivation and destruction of conserved Trp159 of Fe-superoxide dismutase from Porphyromonas gingivalis by hydrogen peroxide. European Journal of Biochemistry, 253, 49–56. doi:10.1046/j.1432-1327.1998.2530049.x.
Bannister, J. V., Bannister, W. H., & Rotilio, G. (1987). Aspects of the structure, function, and applications of superoxide dismutase. CRC Critical Reviews in Biochemistry, 22, 111–180. doi:10.3109/10409238709083738.
Yamakura, F., Sugio, S., Hiraoka, B. Y., Ohmori, D., & Yokota, T. (2003). Pronounced conversion of the metal-specific activity of superoxide dismutase from Porphyromonas gingivalis by the mutation of a single amino acid (Gly155Thr) located apart from the active site. Biochemistry, 42, 10790–10799. doi:10.1021/bi0349625.
Hiraoka, B. Y., Yamakura, F., Sugio, S., & Nakayama, K. (2000). A change of the metal-specific activity of a cambialistic superoxide dismutase from Porphyromonas gingivalis by a double mutation of Gln-70 to Gly and Ala-142 to Gln. The Biochemical Journal, 345, 345–350. doi:10.1042/0264-6021:3450345.
Wintjens, R., Noel, C., May, A. C., Gerbod, D., Dufernez, F., Capron, M., et al. (2004). Specificity and phenetic relationships of iron- and manganese-containing superoxide dismutases on the basis of structure and sequence comparisons. The Journal of Biological Chemistry, 279, 9248–9254. doi:10.1074/jbc.M312329200.
Bunting, K., Cooper, J. B., Badasso, M. O., Tickle, I. J., Newton, M., Wood, S. P., et al. (1998). Engineering a change in metal-ion specificity of the iron-dependent superoxide dismutase from Mycobacterium tuberculosis—X-ray structure analysis of site-directed mutants. European Journal of Biochemistry, 251, 795–803. doi:10.1046/j.1432-1327.1998.2510795.x.
Miller, A. F., Schwartz, A. L., & Vance, C. K. (1999). Mechanism of redox tuning in Fe- and Mn-superoxide dismutase. Journal of Inorganic Biochemistry, 74, 41.
Ludwig, M. L., Metzger, A. L., Pattridge, K. A., & Stallings, W. C. (1991). Manganese superoxide dismutase from Thermus thermophilus. A structural model refined at 1.8 Å resolution. Journal of Molecular Biology, 219, 335–358. doi:10.1016/0022-2836(91)90569-R.
Henne, A., Bruggemann, H., Raasch, C., Wiezer, A., Hartsch, T., Liesegang, H., et al. (2004). The genome sequence of the extreme thermophile Thermus thermophilus. Nature Biotechnology, 22, 547–553. doi:10.1038/nbt956.
Marklund, S., & Marklund, G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry, 47, 469–474. doi:10.1111/j.1432-1033.1974.tb03714.x.
Roth, E. F., Jr, & Gilbert, H. S. (1984). The pyrogallol assay for superoxide dismutase: absence of a glutathione artifact. Analytical Biochemistry, 137, 50–53. doi:10.1016/0003-2697(84)90344-0.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., & Higgins, D. G. (1997). The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25, 4876–4882. doi:10.1093/nar/25.24.4876.
Clamp, M., Cuff, J., Searle, S. M., & Barton, G. J. (2004). The Jalview Java alignment editor. Bioinformatics (Oxford, England), 20, 426–427. doi:10.1093/bioinformatics/btg430.
Sanchez, R., & Sali, A. (2000). Comparative protein structure modeling. Introduction and practical examples with modeller. Methods in Molecular Biology (Clifton, NJ), 143, 97–129.
Laskowski, R. A., MacArthur, M. W., & Thornton, J. M. (1998). Validation of protein models derived from experiment. Current Opinion in Structural Biology, 8, 631–639. doi:10.1016/S0959-440X(98)80156-5.
Kerfeld, C. A., Yoshida, S., Tran, K. T., Yeates, T. O., Cascio, D., Bottin, H., et al. (2003). The 1.6 Å resolution structure of Fe-superoxide dismutase from the thermophilic cyanobacterium Thermosynechococcus elongatus. Journal of Biological Inorganic Chemistry, 8, 707–714. doi:10.1007/s00775-003-0469-0.
Sugio, S., Hiraoka, B. Y., & Yamakura, F. (2000). Crystal structure of cambialistic superoxide dismutase from Porphyromonas gingivalis. European Journal of Biochemistry, 267, 3487–3495. doi:10.1046/j.1432-1327.2000.01373.x.
Choi, M. Y., Cardarelli, L., Therien, A. G., & Deber, C. M. (2004). Non-native interhelical hydrogen bonds in the cystic fibrosis transmembrane conductance regulator domain modulated by polar mutations. Biochemistry, 43, 8077–8083. doi:10.1021/bi0494525.
Therien, A. G., Grant, F. E., & Deber, C. M. (2001). Interhelical hydrogen bonds in the CFTR membrane domain. Nature Structural Biology, 8, 597–601. doi:10.1038/89631.
da Silva, A. C., Kendrick-Jones, J., & Reinach, F. C. (1995). Determinants of ion specificity on EF-hands sites. Conversion of the Ca2+/Mg2+ site of smooth muscle myosin regulatory light chain into a Ca(2+)-specific site. The Journal of Biological Chemistry, 270, 6773–6778. doi:10.1074/jbc.270.12.6773.
Acknowledgments
We will give our sincere thanks to Dr. Hu Zhu (China University of Petroleum, East China) for careful reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, T., Qiu, A., Meng, F. et al. Changing the Metal Binding Specificity of Superoxide Dismutase from Thermus thermophilus HB-27 by a Single Mutation. Mol Biotechnol 42, 146–153 (2009). https://doi.org/10.1007/s12033-009-9149-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-009-9149-9