Abstract
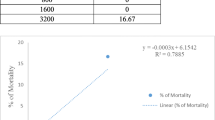
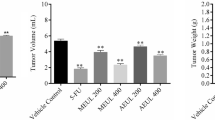
Rhamnetin is a flavonoid which contained in especially clove, such as apple, tea, and onion plant. Rhamnetin has been used in cancer research due to its antitumor and antioxidant properties. In this study, effects of rhamnetin administration at different doses on ascites and solid tumors were investigated in Balb/C mice bearing EAT model that originating from rat breast adenocarcinoma. Experimental procedure: Overall, 92 Balb-c mice were used in this study. EAT cells (1 × 106 cells) that harvested from stock animals were injected to all rats via intraperitoneal and subcutaneous route. Rhamnetin (100 µg/kg–200 µg/kg) were given intraperitoneally and subcutaneously during 10 and 15 days to the animals bearing ascites tumor and solid tumor, respectively. Throughout experiments, weight changes were recorded in all groups. The maximum weight increase was observed in the control group among all groups (ascites and solid tumor groups). In the treatment groups, the least weight increase were determined in 200-µg/kg rhamnetin applied. The lowest increase in tumor volume was observed in the group that received 200-µg/kg rhamnetin (2.84) when compared to tumor control group (3.67). Result and conclusion: We determined that the number of live and dead cells in the treatment groups administered with the mean rhamnetin dose (2.5 µg/ml) was found in the count made in the EAT cell line after the incubation periods. We observed that rhamnetin plays an important role against cancer formation. We have obtained important results in our study, but detailed studies on the relationship between rhamnetin and cancer are needed.



Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- µg:
-
Microgram
- µl:
-
Microliter
- cm:
-
Centimeter
- kg:
-
Kilogram
- g:
-
Gram
- mg:
-
Milligram
- h:
-
Hour
- DAB:
-
Diaminobenzidine
- DAPI:
-
4,6-Diamidino-2-phenylindole
- TdT:
-
Terminal deoxynucleotidyl transferase
- dUTP:
-
Nonisotopic labeled nucleotides
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP Nick-end Labeling
- EAT:
-
Ehrlich Ascites Tumor
- PS:
-
Physiological saline
- FITCH:
-
Fluorescein isothiocyanate
- FVIII:
-
Factor VIII
- ml:
-
Milliliter
- mm3 :
-
Cubic millimeter
- NSCLC:
-
Non-small cell lung cancer cells
- PBS:
-
Phosphate buffer saline
- DNA:
-
Deoxyribonucleic acid
- W:
-
Watt
- ip:
-
Intraperitoneal
- sc:
-
Subcutaneous
References
Imran M, Rauf A, Izneid TA, et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed Pharmacother. 2019;112:108612. https://doi.org/10.1016/j.biopha.2019.108612.
Srivastava S, Somasagara RR, Hegde M, et al. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep. 2016;6:24049.
WHO Report On Cancer Settıng Priorities, Investiıng Wisely And Providing Care For All;2020. Chapter 01, The Growing Burden Of Cancer P.13
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. Ca Cancer J Clin. 2020;70:7–30.
Raju A, Christina MJA, Murali A. Antitumor activity of ethanol and aqueous extracts of Drosera Burmannii Vahl. EAC bearing mice Spatula DD. 2012;2:83–8.
Tavsan Z, Kayali HA. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed Pharmacother. 2019;116:109004.
Yılmaz S, Ülger H, Ertekin T, et al. Investigating the anti-tumoral effect of curcumin on the mice in which Ehrlich ascites and solid tumor is created. Iran J Basic Med Sci. 2019;22(4):418–25.
Youns M, Halim Hegazy WA. The Natural flavonoid fisetin inhibits cellular proliferation of hepatic, colorectal, and pancreatic cancer cells through modulation of multiple signaling pathways. PLoS ONE. 2017;12(1):e0169335.
Lee KP, Kim JE, Park WH. Cytoprotective effect of rhamnetin on miconazoleinduced H9c2 cell damage. Nutr Res Pract. 2015;9:586–91.
Babasaheb PB, Shrikant SG, Ragini GB, et al. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-infl ammatory and antioxidant agents. Bioorg Med Chem. 2010;18:1364–70.
Varmus H. The new era in cancer research. Science. 2006;26:1162–5.
Tozkoparan B, Aytaç SP. As a therapeutic target in cancer chemotherapy, glutathione S-transferases. J Fac Pharm. 2007;27:139–64.
Kızılcı S. Factors affecting the quality of life of cancer patients and their relatives receiving chemotherapy. J Nurs. 1999;3:18–26.
Videira M, Reis RL, Brito MA. Deconstructing breast cancer cell biology and the mechanisms of multidrug resistance. Biochim Biophys Acta. 2014;1846:312–25.
Zeybek Ü. Cancer research and experimental models. J Exp Med Res Ins. 2013;3:187–98.
Tsaı PH, Cheng CH, Lın CY, et al. Dietary flavonoids luteolin and quercetin suppressed cancer stem cell properties and metastatic potential of isolated prostate cancer cells. Anticancer Res. 2016;36(12):6367–80.
Kim YJ. Rhamnetin attenuates melanogenesis by suppressing oxidative stress and pro-inflammatory mediators. Biol Pharm Bull. 2013;36(8):1341–7.
Kang JH, Kim EG, Kim W, et al. Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by miR-34a-mediated suppression of notch-1 expression in non-small cell lung cancer cell lines. J Biol Chem. 2013;288(38):27343–57.
Lan L, Wang Y, Pan Z, et al. Rhamnetin induces apoptosis in human breast cancer cells via the miR-34a/Notch-1 signaling pathway. Oncol Lett. 2019;17(1):676–82.
Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst. 2002;94:883–93.
Yılmaz H, Ertekin T, Atay E, et al. Antioxidant role of melatonin against nicotine’s teratogenic effects on embryonic bone development. Iran J Basic Med Sci. 2018;21:787–93.
Delisser HM. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–7.
Yokozawa T, Kim YJ. Piceatannol inhibits melanogenesis by its antioxidative actions. Biol Pharm Bull. 2007;30(11):2007–11. https://doi.org/10.1248/bpb.30.2007.
Kim YJ, Kang KS, Yokozawa T. The anti-melanogenic effect of pycnogenol by its anti-oxidative actions. Food Chem Toxicol. 2008;46(7):2466–71. https://doi.org/10.1016/j.fct.2008.04.002.
Kim YJ. Antimelanogenic and antioxidant properties of gallic acid. Biol Pharm Bull. 2007;30(6):1052–5. https://doi.org/10.1248/bpb.30.1052.
Chung SW, Ha YM, Kim YJ, et al. Inhibitory effects of 6-(3-hydroxyphenyl)-2-naphthol on tyrosinase activity and melanin synthesis. Arch Pharm Res. 2009;32(2):289–94. https://doi.org/10.1007/s12272-009-1235-9.
Panich U, Onkoksoong T, Limsaengurai S, et al. UVA-induced melanogenesis and modulation of glutathione redox system in different melanoma cell lines: the protective effect of gallic acid. J Photochem Photobiol B. 2012;1(108):16–22. https://doi.org/10.1016/j.jphotobiol.2011.12.004.
Bağcı Uzun G, Nisari M, Hanım Yay A, et al. Investigating the anti-tumoral effect of yarrow (Achillea milllefolium) on the mice in which ehrlich solid tumor is created. Med Oncol. 2023;40:42. https://doi.org/10.1007/s12032-022-01917-3.
Acknowledgements
This research was produced from Özlem Bozkurt’s PhD thesis named “The investigating the antitumoral effect of rhamnetin on the mice,” in which Ehrlich ascites and solid tumor is created. Financial source of this research was funded by Erciyes University Scientific Research Projects Coordination Unit.
Funding
Bilimsel Araştırma Projeleri, Erciyes Üniversitesi, TDK-2014-5247, Erdoğan Unur
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bozkurt, Ö., Yılmaz, S., Alpa, Ş. et al. Investigation of the effect of rhamnetin on mice injected with solid and ehrlich ascites tumor. Med Oncol 40, 124 (2023). https://doi.org/10.1007/s12032-023-01981-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-01981-3