Abstract
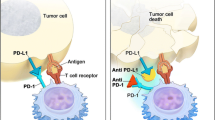
This study aimed to investigate the efficacy of Everolimus (EVE) in combination with immune checkpoint inhibitors (ICIs) in bladder cancer treatment and the underlying mechanisms. In vitro, MB49 cells were exposed to gradient concentrations (0 nM–100 nM) of EVE for 48 h, to investigate the cell viability and cell proliferative potential. In vivo, we applied a subcutaneous tumor mouse model of bladder cancer and the mice were treated with EVE monotherapy (different doses) or in combination with anti-programmed cell death protein 1 (PD-1) agents to study the impacts on tumor growth and explore the immune mechanism. The influences of treatments on peripheral immune profiles and tumor immune microenvironment were also discussed. EVE could inhibit the growth of MB49 cells in vitro. Though high-dose EVE monotherapy could induce tumor regression in vivo, it also contributed to immunosuppression. High-dose EVE inhibited the expression of PD-L1 by inhibiting Th1 cytokine secretion, while combined therapy with PD-1 inhibitors showed no extra profit. Low-dose EVE in combination with PD-1 inhibitors could effectively suppress tumor growth by increasing periphery CD8+ T cell frequency and GZMB+ CD8+ T cell frequency in the tumor microenvironment. High-dose EVE monotherapy induced tumor regression, but with immunosuppression to some content. Combination therapy with low-dose EVE and PD-1 inhibitor could effectively inhibit the growth of bladder tumors by enhancing the antitumor immunity of CD8+ T cells in both periphery and tumor microenvironment.




Similar content being viewed by others
Data availability
The datasets used in this study are available from the corresponding author upon reasonable request.
Code availability
Not appliable.
References
Pinto-Leite R, Arantes-Rodrigues R, Sousa N, Oliveira PA, Santos L: mTOR inhibitors in urinary bladder cancer. (1423–0380 (Electronic)).
Fujii Y: Prediction models for progression of non-muscle-invasive bladder cancer: A review. (1442–2042 (Electronic)).
Kobayashi T: Understanding the biology of urothelial cancer metastasis. (2214–3882 (Print)).
Hafeez S, Horwich A, Omar O, Mohammed K, Thompson A, Kumar P, Khoo V, Van As N, Eeles R, Dearnaley D et al: Selective organ preservation with neo-adjuvant chemotherapy for the treatment of muscle invasive transitional cell carcinoma of the bladder. (1532–1827 (Electronic)).
Stenzl A, Cowan Nc Fau - De Santis M, De Santis M Fau - Kuczyk MA, Kuczyk Ma Fau - Merseburger AS, Merseburger As Fau - Ribal MJ, Ribal Mj Fau - Sherif A, Sherif A Fau - Witjes JA, Witjes JA: Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. (1873–7560 (Electronic)).
Elsasser J, Janssen MW, Becker F, Suttmann H, Schmitt K, Sester U, Stockle M, Sester M: Antigen-specific CD4 T cells are induced after intravesical BCG-instillation therapy in patients with bladder cancer and show similar cytokine profiles as in active tuberculosis. PLoS One 2013, 8(9):e69892.
Tian T, Li X, Zhang J: mTOR Signaling in Cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. LID - https://doi.org/10.3390/ijms20030755 [doi] LID - 755. (1422–0067 (Electronic)).
Justin S, Rutz J, Maxeiner S, Chun FK, Juengel E, Blaheta RA: Bladder Cancer Metastasis Induced by Chronic Everolimus Application Can Be Counteracted by Sulforaphane In Vitro. LID - https://doi.org/10.3390/ijms21155582 [doi] LID - 5582. (1422–0067 (Electronic)).
Vasconcelos-Nóbrega C, Pinto-Leite R Fau - Arantes-Rodrigues R, Arantes-Rodrigues R Fau - Ferreira R, Ferreira R Fau - Brochado P, Brochado P Fau - Cardoso ML, Cardoso Ml Fau - Palmeira C, Palmeira C Fau - Salvador A, Salvador A Fau - Guedes-Teixeira CI, Guedes-Teixeira Ci Fau - Colaço A, Colaço A Fau - Palomino LF et al: In vivo and in vitro effects of RAD001 on bladder cancer. (1873–2496 (Electronic)).
Milowsky MI, Iyer G Fau - Regazzi AM, Regazzi Am Fau - Al-Ahmadie H, Al-Ahmadie H Fau - Gerst SR, Gerst Sr Fau - Ostrovnaya I, Ostrovnaya I Fau - Gellert LL, Gellert Ll Fau - Kaplan R, Kaplan R Fau - Garcia-Grossman IR, Garcia-Grossman Ir Fau - Pendse D, Pendse D Fau - Balar AV et al: Phase II study of everolimus in metastatic urothelial cancer. (1464–410X (Electronic)).
Seront E, Rottey S, Sautois B, Kerger J, D'Hondt LA, Verschaeve V, Canon JL, Dopchie C, Vandenbulcke JM, Whenham N et al: Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: clinical activity, molecular response, and biomarkers. (1569–8041 (Electronic)).
Meric-Bernstam F, Gonzalez-Angulo AM: Targeting the mTOR signaling network for cancer therapy. (1527–7755 (Electronic)).
Schreiber RD, Old Lj Fau - Smyth MJ, Smyth MJ: Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. (1095–9203 (Electronic)).
Rouanne M, Roumiguié M, Houédé N, Masson-Lecomte A, Colin P, Pignot G, Larré S, Xylinas E, Rouprêt M, Neuzillet Y: Development of immunotherapy in bladder cancer: present and future on targeting PD(L)1 and CTLA-4 pathways. (1433–8726 (Electronic)).
Patel SP, Kurzrock R: PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. (1538–8514 (Electronic)).
Rouanne M, Loriot Y, Lebret T, Soria JC: Novel therapeutic targets in advanced urothelial carcinoma. (1879–0461 (Electronic)).
Jin X, Ma J, Liang X, Tang K, Liu Y, Yin X, Zhang Y, Zhang H, Xu P, Chen D, et al. Pre-instillation of tumor microparticles enhances intravesical chemotherapy of nonmuscle-invasive bladder cancer through a lysosomal pathway. Biomaterials. 2017;113:93–104.
Hirayama Y, Gi M, Yamano S, Tachibana H, Okuno T, Tamada S, Nakatani T, Wanibuchi H. Anti-PD-L1 treatment enhances antitumor effect of everolimus in a mouse model of renal cell carcinoma. Cancer Sci. 2016;107(12):1736–44.
Zhang C, Duan Y, Xia M, Dong Y, Chen Y, Zheng L, Chai S, Zhang Q, Wei Z, Liu N, et al. TFEB mediates immune evasion and resistance to mTOR inhibition of renal cell carcinoma via induction of PD-L1. Clin Cancer Res. 2019;25(22):6827–38.
Wang C, Yi T, Qin L, Maldonado RA, von Andrian UH, Kulkarni S, Tellides G, Pober JS. Rapamycin-treated human endothelial cells preferentially activate allogeneic regulatory T cells. J Clin Invest. 2013;123(4):1677–93.
Deng L, Qian G, Zhang S, Zheng H, Fan S, Lesinski GB, Owonikoko TK, Ramalingam SS, Sun S-Y. Inhibition of mTOR complex 1/p70 S6 kinase signaling elevates PD-L1 levels in human cancer cells through enhancing protein stabilization accompanied with enhanced beta-TrCP degradation. Oncogene. 2019;38(35):6270–82.
Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76(2):227–38.
Zhang Q, Zhang Y, Chen Y, Qian J, Zhang X, Yu K. A novel mTORC1/2 inhibitor (MTI-31) inhibits tumor growth, epithelial-mesenchymal transition, metastases, and improves antitumor immunity in preclinical models of lung cancer. Clin Cancer Res. 2019;25(12):3630–42.
Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–44.
Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–12.
Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–8.
Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177(2):944–9.
Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL-4–induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101(11):4457–63.
Moore EC, Cash HA, Caruso AM, Uppaluri R, Hodge JW, Van Waes C, Allen CT. Enhanced tumor control with combination mTOR and PD-L1 inhibition in syngeneic oral cavity cancers. Cancer Immunol Res. 2016;4(7):611–20.
Acknowledgements
We thank Dr. Longcheng Li for sharing the murine tumor cell line MB49.
Funding
This work was supported by the Science and Technology Commission of Shanghai Municipality (No. 21511902300).
Author information
Authors and Affiliations
Contributions
Weimin Xia: study design, animal experinment, data analysis, and manuscript writing. Shun Zhang: study design, animal experinment, and manuscript writing. Huangqi Duan: animal experinment and data analysis. Chen Wang: animal experinment and data analysis. Subo Qian: data analysis, manuscript writing and editing, and revision study design. Haibo Shen: study design, data analysis, manuscript editing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of Xinhua Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xia, W., Zhang, S., Duan, H. et al. The combination therapy of Everolimus and anti-PD-1 improves the antitumor effect by regulating CD8+ T cells in bladder cancer. Med Oncol 39, 37 (2022). https://doi.org/10.1007/s12032-021-01624-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01624-5