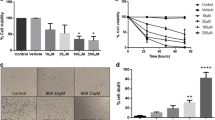
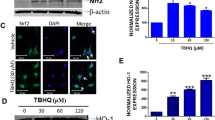
Abstract
Gliomas are one of the most aggressive brain tumors with a poor prognosis in the central nervous system. Bexarotene is a third-generation retinoid X receptor agonist that is promising in the treatment of both cancer and neurodegenerative diseases. In this study, we aimed to investigate the cytotoxic and anti-proliferative effects of bexarotene in C6 glioma cells through the PPARγ/NF-κB pathway. In the study, first cytotoxic bexarotene concentrations for C6 cells were detected, and then apoptosis profile, reactive oxygen species (ROS), total antioxidant (TAS), 8-hydroxy-2′-deoxyguanosine (8-OHdG) and nuclear factor-κB (NF-κB) levels in the cells were determined. In addition, peroxisome proliferator-activated receptor γ (PPARγ) mRNA expression analysis was carried out. As a result, we detected concentration- and time-dependent antiproliferative effects of bexarotene on C6 cells. We found that bexarotene treatment decreased NF-κB and TAS levels and increased PPARγ and 8-OHdG levels in C6 cells. Bexarotene enhanced PPARγ expression in a dose-dependent manner when compared to the control group (P < 0.01). Furthermore, we determined that bexarotene-induced apoptotic C6 cells enhanced through Annexin V-FITC/PI staining and caspase-3/-7 activation analyses since phosphatidylserine level on the outer surface of the cell membrane and caspase-3/-7 activities were increased in the cells treated with bexarotene. In conclusion, bexarotene treatment in C6 glioma cells could modulate apoptosis profile, DNA damage, ROS production, and reduction of TAS levels through inhibition of NF-κB by enhancing PPARγ expression.






Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–50. https://doi.org/10.1001/jama.2013.280319.
Wirsching HG, Galanis E, Weller M. Glioblastoma. Handb Clin Neurol. 2016;134:381–97. https://doi.org/10.1016/B978-0-12-802997-8.00023-2.
Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392(10145):432–46. https://doi.org/10.1016/S0140-6736(18)30990-5.
Grobben B, De Deyn PP, Slegers H. Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res. 2002;310(3):257–70. https://doi.org/10.1007/s00441-002-0651-7.
Farol LT, Hymes KB. Bexarotene: a clinical review. Expert Rev Anticancer Ther. 2004;4(2):180–8. https://doi.org/10.1586/14737140.4.2.180.
Qu L, Tang X. Bexarotene: a promising anticancer agent. Cancer Chemother Pharmacol. 2010;65(2):201–5. https://doi.org/10.1007/s00280-009-1140-4.
Hong F, Pan S, Guo Y, Xu P, Zhai Y. PPARs as Nuclear Receptors for Nutrient and Energy Metabolism. Molecules. 2019;24(14):2545. https://doi.org/10.3390/molecules24142545.
Brunmeir R, Xu F. Functional Regulation of PPARs through Post-Translational Modifications. Int J Mol Sci. 2018;19(6):1738. https://doi.org/10.3390/ijms19061738.
Li J, Liu YP. The roles of PPARs in human diseases. Nucleosides Nucleotides Nucleic Acids. 2018;37(7):361–82. https://doi.org/10.1080/15257770.2018.1475673.
Wang P, Li B, Cai G, Huang M, Jiang L, Pu J, Li L, Wu Q, Zuo L, Wang Q, Zhou P. Activation of PPAR-γ by pioglitazone attenuates oxidative stress in aging rat cerebral arteries through upregulating UCP2. J Cardiovasc Pharmacol. 2014;64(6):497–506. https://doi.org/10.1097/FJC.0000000000000143.
Li CC, Yang HT, Hou YC, Chiu YS, Chiu WC. Dietary fish oil reduces systemic inflammation and ameliorates sepsis-induced liver injury by up-regulating the peroxisome proliferator-activated receptor gamma-mediated pathway in septic mice. J Nutr Biochem. 2014;25(1):19–25. https://doi.org/10.1016/j.jnutbio.2013.08.010.
Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–24. https://doi.org/10.1038/nri.2017.142.
Chen GG, Lee JF, Wang SH, Chan UP, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and NF-kappaB in human colon cancer. Life Sci. 2002;70(22):2631–46. https://doi.org/10.1016/s0024-3205(02)01510-2.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. https://doi.org/10.1016/j.cell.2010.01.025.
Joyce D, Albanese C, Steer J, Fu M, Bouzahzah B, Pestell RG. NF-kappaB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 2001;12(1):73–90. https://doi.org/10.1016/s1359-6101(00)00018-6.
Janssens S, Tschopp J. Signals from within: the DNA-damage-induced NF-kappaB response. Cell Death Differ. 2006;13(5):773–84. https://doi.org/10.1038/sj.cdd.4401843.
Shen D, Yu X, Wu Y, Chen Y, Li G, Cheng F, Xia L. Emerging roles of bexarotene in the prevention, treatment and anti-drug resistance of cancers. Expert Rev Anticancer Ther. 2018;18(5):487–99. https://doi.org/10.1080/14737140.2018.
Evans RM, Mangelsdorf DJ. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014;157(1):255–66. https://doi.org/10.1016/j.cell.2014.03.012.
Wang L, DeMarco SS, Chen J, Phillips CM, Bridges LC. Retinoids Bias Integrin Expression and Function in Cutaneous T-Cell Lymphoma. J Invest Dermatol. 2015;135(8):2102–8. https://doi.org/10.1038/jid.2015.122.
Wu K, DuPre E, Kim H, Tin-U CK, Bissonnette RP, Lamph WW, Brown PH. Receptor-selective retinoids inhibit the growth of normal and malignant breast cells by inducing G1 cell cycle blockade. Breast Cancer Res Treat. 2006;96(2):147–57. https://doi.org/10.1007/s10549-005-9071-1.
Zhang C, Hazarika P, Ni X, Weidner DA, Duvic M. Induction of apoptosis by bexarotene in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. Clin Cancer. 2002;8(5):1234–40.
Huuskonen MT, Loppi S, Dhungana H, Keksa-Goldsteine V, Lemarchant S, Korhonen P, Wojciechowski S, Pollari E, Valonen P, Koponen J, Takashima A, Landreth G, Goldsteins G, Malm T, Koistinaho J, Kanninen KM. Bexarotene targets autophagy and is protective against thromboembolic stroke in aged mice with tauopathy. Sci Rep. 2016;6:33176. https://doi.org/10.1038/srep33176.
Ai X, Mao F, Shen S, Shentu Y, Wang J, Lu S. Bexarotene inhibits the viability of non-small cell lung cancer cells via slc10a2/PPARγ/PTEN/mTOR signaling pathway. BMC Cancer. 2018;18(1):407. https://doi.org/10.1186/s12885-018-4224-x.
Heo JC, Jung TH, Lee S, Kim HY, Choi G, Jung M, Jung D, Lee HK, Lee JO, Park JH, Hwang D, Seol HJ, Cho H. Effect of bexarotene on differentiation of glioblastoma multiforme compared with ATRA. Clin Exp Metastasis. 2016;33(5):417–29. https://doi.org/10.1007/s10585-016-9786-x.
Hanafi R, Anestopoulos I, Voulgaridou GP, Franco R, Georgakilas AG, Ziech D, Malamou-Mitsi V, Pappa A, Panayiotidis MI. Oxidative stress based-biomarkers in oral carcinogenesis: how far have we gone? Curr Mol Med. 2012;12(6):698–703. https://doi.org/10.2174/156652412800792598.
Klaunig JE. Oxidative Stress and Cancer. Curr Pharm Des. 2018;24(40):4771–8. https://doi.org/10.2174/1381612825666190215121712.
Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64. https://doi.org/10.1016/j.semcdb.2017.05.023.
Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–96. https://doi.org/10.2741/1667.
Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–47. https://doi.org/10.1038/nrd4002.
Khoo NK, Hebbar S, Zhao W, Moore SA, Domann FE, Robbins ME. Differential activation of catalase expression and activity by PPAR agonists: implications for astrocyte protection in anti-glioma therapy. Redox Biol. 2013;1(1):70–9. https://doi.org/10.1016/j.redox.2012.12.006.
Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(2):120–39. https://doi.org/10.1080/10590500902885684.
Al Mamun Bhuyan A, Bissinger R, Cao H, Lang F. Triggering of Suicidal Erythrocyte Death by Bexarotene. Cell Physiol Biochem. 2016;40(5):1239–51. https://doi.org/10.1159/000453178.
Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–6. https://doi.org/10.1038/35037710.
Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–19. https://doi.org/10.1016/s0092-8674(04)00046-7.
Zander T, Kraus JA, Grommes C, Schlegel U, Feinstein D, Klockgether T, Landreth G, Koenigsknecht J, Heneka MT. Induction of apoptosis in human and rat glioma by agonists of the nuclear receptor PPARgamma. J Neurochem. 2002;81(5):1052–60. https://doi.org/10.1046/j.1471-4159.2002.00899.x.
Liu Y, Meng Y, Li H, Li J, Fu J, Liu Y, Chen XG. Growth inhibition and differentiation induced by peroxisome proliferator activated receptor gamma ligand rosiglitazone in human melanoma cell line A375. Med Oncol. 2006;23(3):393–402. https://doi.org/10.1385/mo:23:3:393.
Gretskaya NM, Gamisonia AM, Dudina PV, Zakharov SS, Sherstyanykh G, Akasov R, Burov S, Serkov IV, Akimov MG, Bezuglov VV, Markvicheva E. Novel bexarotene derivatives: Synthesis and cytotoxicity evaluation for glioma cells in 2D and 3D in vitro models. Eur J Pharmacol. 2020;883:173346. https://doi.org/10.1016/j.ejphar.2020.173346.
Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–35. https://doi.org/10.1146/annurev.med.53.082901.104018.
Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001;169(3):453–9. https://doi.org/10.1677/joe.0.1690453.
Hwang JK, Yu HN, Noh EM, Kim JM, Hong OY, Youn HJ, Jung SH, Kwon KB, Kim JS, Lee YR. DHA blocks TPA-induced cell invasion by inhibiting MMP-9 expression via suppression of the PPAR-γ/NF-κB pathway in MCF-7 cells. Oncol Lett. 2017;13(1):243–9. https://doi.org/10.3892/ol.2016.5382.
Kiekow CJ, Figueiro F, Dietrich F, Vechia LD, Pires EN, Jandrey EH, Gnoatto SC, Salbego CG, Battastini AM, Gosmann G. Quercetin derivative induces cell death in glioma cells by modulating NF-κB nuclear translocation and caspase-3 activation. Eur J Pharm Sci. 2016;84:116–22. https://doi.org/10.1016/j.ejps.2016.01.019.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by CH, FK and SK. The first draft of the manuscript was written by CH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hacioglu, C., Kar, F., Kacar, S. et al. Bexarotene inhibits cell proliferation by inducing oxidative stress, DNA damage and apoptosis via PPARγ/ NF-κB signaling pathway in C6 glioma cells. Med Oncol 38, 31 (2021). https://doi.org/10.1007/s12032-021-01476-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01476-z