Abstract
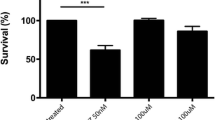
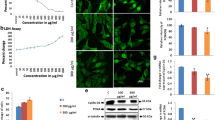
Salicylidene acylhydrazide group synthetic compounds ME0053, ME005 and ME0192 are known for their iron chelating properties and due to these properties they are primarily used for blocking the bacterial type 3 secretory virulence system. On the other side, targeting the metabolic pathways of iron can provide new tools for cancer prognosis and treatment. Therefore, in this study, considering their iron chelating function, the effects of the compounds ME0053, ME0055 and ME0192 were investigated in SH-SY5Y neuroblastoma cell line. Iron chelating compounds are generally known to be effective in tumor development and metastasis by targeting iron in the cell. They can exert this effect through molecules such as cyclin, CDKs, as well as signaling pathways such as PI3K/AKT and ERK/MAPK. For this reason, we analyzed the effect of the iron chelating compounds of ME0053, ME0055 and ME0192 on cell viability and proliferation rate both through ERK/MAPK and PI3K/AKT signal paths, and through the oncogenic Speedy/RINGO protein that is likely to have a regulatory effect on these two signaling pathways. Apoptosis was also investigated by measuring the amount of active caspase-3, an apoptotic marker. Along with the decrease observed in the Speedy/RINGO level, it was observed that the PI3K/AKT and ERK/MAPK signaling were decreased. This suggests that ME0053, ME0055 and ME0192 compounds significantly decrease the Speedy/RINGO expression which has a regulatory effect on the ERK/MAPK and PI3K/AKT signaling. Besides, analyzing active caspase-3 levels showed that the compounds ME0053, ME0055 and ME0192 increased its level by 218%, 60% and 175% in SH-SY5Y cells, respectively. The results of this study will pave the way for better understanding of the regulation of cancer-related ERK/MAPK and PI3K/AKT pathways and the oncogenic Speedy/RINGO which potentially affects these pathways, through synthetic salicylidene acylhydrazides and their therapeutic use in cancer.





Similar content being viewed by others
References
Hung DT, Rubin EJ. Chemical biology and bacteria: not simply a matter of life or death. Curr Opin Chem Biol. 2006;10:321–6.
Slepenkin A, Enquist PA, Hagglund U, de La Maza LM, Elofsson M, et al. Reversal of the anti chlamydial activity of putative type III secretion inhibitors by iron. Infect Immun. 2007;75:3478–89.
Tree JJ, Wang D, McInally C, Mahajan A, Layton A, et al. Characterization of the effects of salicylidene acylhydrazide compounds on type III secretion in Escherichia coli O157:H7. Infect Immun. 2009;77:4209–20.
Richardson D, Ponka P, Baker E. The effect of the iron (III) chelator, desferrioxamine, on iron and transferrin uptake by the human malignant melanoma cell. Cancer Res. 1994;54:685–9.
Merlot AM, Kalinowski DS, Richardson DR. Novel chelators for cancer treatment: where are we now? Antioxid Redox Signal. 2013;18:973–1006.
Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 2012;40:139–46.
Vara JAF, Casado E, Castro JD, Cejas P, Belda-Iniesta C, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204.
Kovacevic Z, Chikhani S, Lui GY, Sivagurunathan S, Richardson DR. The iron-regulated metastasis suppressor NDRG1 targets NEDD4L, PTEN, and SMAD4 and inhibits the PI3K and Ras signaling pathways. Antioxid Redox Signal. 2013;18:874–87.
Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35.
Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90.
Hubert RS, Vivanco I, Chen E, Rastegar S, Leong K, et al. STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci USA. 1999;96:14523–8.
Yildiz A, Tanriverdi O. MAPK and AKT pathway intersection in neuroblastoma cells. Curr Trends Biomed Eng Biosci. 2017;2:1–4.
Kaneko Y, Kanda N, Maseki N, Sakurai M, Tsuchida Y, et al. Different karyotypic patterns in early and advanced stage neuroblastomas. Cancer Res. 1987;47:311–8.
Cao Z, Liao Q, Su M, Huang K, Jin J, et al. Akt and Erk dual inhibitors: the way forward. Cancer Lett. 2019;459:30–40.
Lubanska D, Porter LA. The atypical cell cycle regülator Spy1 suppresses differentiation of the neuroblastoma stem cell population. Oncoscience. 2014;1:336–48.
Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–13.
Golipour A, Myers D, Seagroves T, Murphy D, Evan GI, et al. The Spy1/RINGO family represents a novel mechanism regulating mammary growth and tumorigenesis. Cancer Res. 2008;68:3591–600.
Liu P, Begley M, Michowski W, Inuzuka H, Ginzberg M, et al. Cell cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014;508:541–5.
Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–62.
Sun J, Zhang D, Zheng Y, Zhao Q, Zheng M, et al. Targeting the metastasis suppressor, NDRG1, using novel iron chelators: regulation of stress fiber-mediated tumor cell migration via modulation of the ROCK1/pMLC2 signaling pathway. Mol Pharmacol. 2013;83:454–69.
Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–73.
Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–50.
Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310.
Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–93.
Eisenstein RS, Blemings KP. Iron regulatory proteins, iron responsive elements and iron homeostasis. J Nutr. 1998;128:2295–8.
Hentze MW, Kuhn LC. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci USA. 1996;93:8175–82.
Wang D, Zetterström CE, Gabrielsen M, Beckham KSH, Tree JJ, et al. Identification of bacterial target proteins for the salicylidene acyl hydrazide Class of virulence-blocking compounds. J Biol Chem. 2011;286:29922–31.
Nordfelth R, Kauppi AM, Norberg HA, Wolf-Watz H, Elofsson M. Small-molecule inhibitors specifically targeting type III secretion. Infect Immun. 2005;73:3104–14.
Kim BS, Yoon KH, Oh HM, Choi EY, Kim SW, et al. Involvement of p38 MAP kinase during iron chelator-mediated apoptotic cell death. Cell Immunol. 2002;220:96–106.
Pruitt K, Pruitt WM, Bilter GK, Westwick JK, Der CJ. Raf-independent deregulation of p38 and JNK mitogenactivated protein kinases are critical for Ras transformation. J Biol Chem. 2002;277:31808–17.
Acknowledgements
This study was supported by grant to Aysegul Yıldız from Mugla Sitki Kocman University Scientific Research Project Office, Research and Development Projects (Project Numbers: 17/251 and 17/023). We sincerely thank Prof. Dr. Uygar Halis Tazebay from Gebze Technical University, Department of Molecular Biology and Genetics and Prof. Dr. Arzu Karabay Korkmaz from lstanbul Technical University, Faculty of Science and Letters, Molecular Biology and Genetics Department for allowing us to use their laboratory infrastructure. We would also thank Assoc. Prof. Emin Ilker Medine from Ege University Institute of Nuclear Sciences for his help about providing SH-SY5Y neuroblastoma cell line and to Prof. Dr. Mikael Elofsson from Umeå University for providing all the salicylidene acylhydrazide compounds used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We certify that all of our affiliations with or without financial involvement, within the past 5 years and foreseeable future and, any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript are completely disclosed (e.g., employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, and royalties). “No conflicts of interest”.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arziman, S., Tanriverdi, O., Kucukvardar, S. et al. Salicylidene acylhydrazides attenuate survival of SH-SY5Y neuroblastoma cells through affecting mitotic regulator Speedy/RINGO and ERK/MAPK–PI3K/AKT signaling. Med Oncol 37, 65 (2020). https://doi.org/10.1007/s12032-020-01391-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01391-9