Abstract
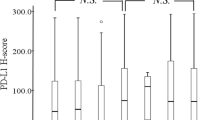
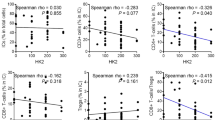
The microenvironment of a tumor may regulate the anti-tumor immune response. Intratumoral acidosis and hypoxia may suppress lymphocyte proliferation and migration, and this may have important implications in modern immunotherapy. The expression of PD-L1 by cancer cells and of PD-1 by tumor infiltrating lymphocytes (TILs) was assessed in tissue specimens from 98 operable NSCLC patients. Their prognostic role and their association with makers of glycolysis and anaerobic metabolism were assessed. Strong cytoplasmic/membrane PD-L1 expression was noted in 45/98 cases. Intense presence of TILs was noted in 42/98 cases (high TIL-score), and intense presence of PD-1 expressing TILs (high PIL-score) in 17/98 cases. PD-L1 expression was directly correlated with high PIL-score (p = 0.005). A significant inverse relationship was found between lactate dehydrogenase LDH5 expression and PIL-score (p = 0.008). Similarly, low PIL-score was significantly linked with high-hexokinase HXKII and monocarboxylate transporter MCT2 expression (p < 0.04). Cases with both intense TIL-score and PIL-score had significantly better survival (p < 0.05). For patients with high TIL-score or high PIL-score, PD-L1 overexpression defined significantly poorer survival (p = 0.01 and p = 0.03, respectively). In multivariate analysis, stage (p = 0.002, HR 3.33, 95%CI 1.4–4.5) and TIL-score (p = 0.02, HR 2.12, 95%CI 1.1–4.0) were independent predictive variables of death events. Given the low specificity of PD-L1 as a biomarker for anti-PD-1/PD-L1 immunotherapy, a combined assessment of TIL, PD-L1, PD-1, and LDH5 provides a tool for an immunological/metabolic classification of NSCLC tumors, with a different prognosis and different expected response to anti-PD-1/PD-L1 immunotherapy, which should be considered in relevant clinical trials.



Similar content being viewed by others
References
Coley WB. The treatment of malignant tumors by repeated innoculations of erysipelas: with a report of ten original cases. Am J Med Sci. 1893;10:487–511.
Bashford EF, Murray JA, Cramer W. The natural and induced resistance of mice to the growth of cancer. Proc R Soc B. 1907;79:164. https://doi.org/10.1098/rspb.1907.0014.
Shi T, Ma Y, Yu L, Jiang J, Shen S, Hou Y, Wang T. Cancer immunotherapy: a focus on the regulation of immune checkpoints. Int J Mol Sci. 2018;2:89. https://doi.org/10.3390/ijms19051389.
Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8.
Leal TA, Ramalingam SS. Immunotherapy in previously treated non-small cell lung cancer (NSCLC). J Thorac Dis. 2018;10(Suppl 3):S422–32.
Udall M, Rizzo M, Kenny J, Doherty J, Dahm S, Robbins P, Faulkner E. PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagn Pathol. 2018;13:12.
Giatromanolaki A, Sivridis E, Arelaki S, Koukourakis MI. Expression of enzymes related to glucose metabolism in non-small cell lung cancer and prognosis. Exp Lung Res. 2017;43:167–74.
Huber V, Camisaschi C, Berzi A, Ferro S, Lugini L, Triulzi T, Tuccitto A, Tagliabue E, Castelli C, Rivoltini. Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol. 2017;43:74–89.
Damaghi M, Wojtkowiak JW, Gillies RJ. pH sensing and regulation in cancer. Front Physiol. 2013;4:370.
Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, Damaghi M, Wojtkowiak JW, Mulé JJ, Ibrahim-Hashim A, Gillies RJ. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 2016;76:1381–90.
Karnik T, Kimler BF, Fan F, Tawfik O. PD-L1 in breast cancer: comparative analysis of 3 different antibodies. Hum Pathol. 2018;72:28–34.
Schwert GW, Winer AD. Lactate dehydrogenase. In: Boyer PD, Lardy HA, Myrback K, editors. The enzymes, vol. 7. New York: Academic Press; 1963. p. 127–48.
Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumor-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103.
Lin G, Fan X, Zhu W, Huang C, Zhuang W, Xu H, Lin X, Hu D, Huang Y, Jiang K, Miao Q, Li C. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocyte in surgically resectable non-small cell lung cancer. Oncotarget. 2017;8:83986–94.
Feng W, Li Y, Shen L, Cai XW, Zhu ZF, Chang JH, Xiang JQ, Zhang YW, Chen HQ, Fu XL. Prognostic value of tumor-infiltrating lymphocytes for patients with completely resected stage IIIA(N2) non-small cell lung cancer. Oncotarget. 2016;7:7227–40.
Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA, Nason KS, Landreneau RJ, Luketich JD, Schuchert MJ. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171:1–5.
Donnem T, Hald SM, Paulsen EE, Richardsen E, Al-Saad S, Kilvaer TK, Brustugun OT, Helland A, Lund-Iversen M, Poehl M, Olsen KE, Ditzel HJ, Hansen O, Al-Shibli K, Kiselev Y, Sandanger TM, Andersen S, Pezzella F, Bremnes RM, Busund LT. Stromal CD8+ T-cell density—a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res. 2015;21:2635–43.
Zhuang X, Xia X, Wang C, Gao F, Shan N, Zhang L, Zhang L. A high number of CD8+ T cells infiltrated in NSCLC tissues is associated with a favorable prognosis. Appl Immunohistochem Mol Morphol. 2010;18:24–8.
Kim MY, Koh J, Kim S, Go H, Jeon YK, Chung DH. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015;88:24–33.
Yan X, Jiao SC, Zhang GQ, Guan Y, Wang JL. Tumor-associated immune factors are associated with recurrence and metastasis in non-small cell lung cancer. Cancer Gene Ther. 2017;24:57–63.
Kinoshita T, Muramatsu R, Fujita T, Nagumo H, Sakurai T, Noji S, Takahata E, Yaguchi T, Tsukamoto N, Kudo-Saito C, Hayashi Y, Kamiyama I, Ohtsuka T, Asamura H, Kawakami Y. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann Oncol. 2016;27:2117–23.
Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98:751–5.
Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–16.
Zhang Y, Wang L, Li Y, Pan Y, Wang R, Hu H, Li H, Luo X, Ye T, Sun Y, Chen H. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther. 2014;7:567–73.
Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M, Yip P, Yu B, O’Toole SA, McCaughan BC, Yearley JH, Horvath LG, Kao S, Boyer M, Scolyer RA. PD-L1 expression is a favorable prognostic factor in early-stage non-small cell carcinoma. Lung Cancer. 2015;89:181–8.
Schmidt LH, Kümmel A, Görlich D, Mohr M, Bröckling S, Mikesch JH, Grünewald I, Marra A, Schultheis AM, Wardelmann E, Müller-Tidow C, Spieker T, Schliemann C, Berdel WE, Wiewrodt R, Hartmann W. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS ONE. 2015;10:e0136023.
Tokito T, Azuma K, Kawahara A, Ishii H, Yamada K, Matsuo N, Kinoshita T, Mizukami N, Ono H, Kage M, Hoshino T. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7–14.
Sun JM, Zhou W, Choi YL, Choi SJ, Kim SE, Wang Z, Dolled-Filhart M, Emancipator K, Wu D, Weiner R, Frisman D, Kim HK, Choi YS, Shim YM, Kim J. Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol. 2016;11:1003–11.
Ameratunga M, Asadi K, Lin X, Walkiewicz M, Murone C, Knight S, Mitchell P, Boutros P, John T. PD-L1 and tumor infiltrating lymphocytes as prognostic markers in resected NSCLC. PLoS ONE. 2016;11:e0153954.
Mori S, Motoi N, Ninomiya H, Matsuura Y, Nakao M, Mun M, Okumura S, Nishio M, Morikawa T, Ishikawa Y. High expression of programmed cell death 1 ligand 1 in lung adenocarcinoma is a poor prognostic factor particularly in smokers and wild-type epidermal growth-factor receptor cases. Pathol Int. 2017;67:37–44.
Okita R, Maeda A, Shimizu K, Nojima Y, Saisho S, Nakata M. PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunol Immunother. 2017;66:865–76.
Wang K, Wang J, Wei F, Zhao N, Yang F, Ren X. Expression of TLR4 in non-small cell lung cancer is associated with PD-L1 and poor prognosis in patients receiving pulmonectomy. Front Immunol. 2017;8:456.
Okuma Y, Hishima T, Kashima J, Homma S. High PD-L1 expression indicates poor prognosis of HIV-infected patients with non-small cell lung cancer. Cancer Immunol Immunother. 2018;67:495–505.
He Y, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, Yu H, Zhou C, Hirsch FR. PD-1, PD-L1 protein expression in non-small cell lung cancer and their relationship with tumor-infiltrating lymphocytes. Med Sci Monit. 2017;23:1208–16.
Dovedi SJ, Illidge TM. The anti-tumor immune response generated by fractionated radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. Oncoimmunology. 2015;4(7):e1016709.
Shen MJ, Xu LJ, Yang L, Tsai Y, Keng PC, Chen Y, Lee SO, Chen Y. Radiation alters PD-L1/NKG2D ligand levels in lung cancer cells and leads to immune escape from NK cell cytotoxicity via IL-6-MEK/Erk signaling pathway. Oncotarget. 2017;8(46):80506–20.
Shen X, Zhang L, Li J, Li Y, Wang Y, Xu ZX. Recent findings in the regulation of programmed death ligand 1 expression. Front Immunol. 2019;14(10):1337.
Koukourakis MI, Giatromanolaki A. Warburg effect, lactate dehydrogenase and radio/chemo-therapy efficacy. Int J Radiat Biol. 2018;18:1–55.
Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90.
Schiltz PM, Dillman RO, Korse CM, Cubellis JM, Lee GJ, De Gast GC. Lack of elevation of serum S100B in patients with metastatic melanoma as a predictor of outcome after induction with an autologous vaccine of proliferating tumor cells and dendritic cells. Cancer Biother Radiopharm. 2008;23:214–21.
Koukourakis MI, Giatromanolaki A, Bougioukas G, Sivridis E. Lung cancer: a comparative study of metabolism related protein expression in cancer cells and tumor associated stroma. Cancer Biol Ther. 2007;6:1476–9.
Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66:632–7.
Loeffler DA, Juneau PL, Masserant S. Influence of tumor physico-chemical conditions on interleukin-2 stimulated lymphocyte proliferation. Br J Cancer. 1992;66:619–22.
Severin T, Muller B, Giese G, Uhl B, Wolf B, Hauschildt S, Kreutz W. pH-dependent LAK cell cytotoxicity. Tumor Biol. 1994;15:304–10.
Loeffler DA, Juneau PL, Heppner GH. Natural killer cell activity under conditions reflective of tumor micro-environment. Int J Cancer. 1991;48:895–9.
Redegeld F, Filippini A, Sitkovsky M. Comparative studies of the cytotoxic T lymphocyte-mediated cytotoxicity and of extracellular ATP-induced cell lysis. Different requirements in extracellular Mg1 and pH. J Immunol. 1991;147:3638–45.
Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, Cova A, Canese R, Jachetti E, Rossetti M, Huber V, Parmiani G, Generoso L, Santinami M, Borghi M, Fais S, Bellone M, Rivoltini L. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72:2746–56.
Bidani A, Wang CZ, Saggi SJ, Heming TA. Evidence for pH sensitivity of tumor necrosis factor-a release by alveolar macrophages. Lung. 1988;176:111–21.
Funding
The study has been funded by the Tumour and Angiogenesis Research Group, Heraklion, Greece
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was obtained from the Internal Scientific Committee and the Ethic Research Committee of the University Hospital of Alexandroupolis (study Approval Number ES11-26-11-18). The study was conducted according to the criteria set by the declaration of Helsinki.
Informed consent
The study is retrospective on ‘existing holdings’ and no informed consent is demanded for examining anonymously archival material (material archived between 2002 and 2007). (Human Tissue Authority, E Research, Code of Practice and standards; https://www.hta.gov.uk/sites/default/files/Code%20E%20-%20Research%20Final.pdf; page 15, Consent exceptions paragraph 56 and 57).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giatromanolaki, A., Koukourakis, I.M., Balaska, K. et al. Programmed death-1 receptor (PD-1) and PD-ligand-1 (PD-L1) expression in non-small cell lung cancer and the immune-suppressive effect of anaerobic glycolysis. Med Oncol 36, 76 (2019). https://doi.org/10.1007/s12032-019-1299-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-019-1299-4