Abstract
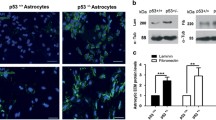
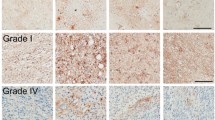
The complex microenvironment of malignant gliomas plays a dynamic and usually cancer-promoting role in glioma progression. Astrocytes, the major stromal cells in the brain, can be activated by glioma microenvironment, resulting in a layer of reactive astrocytes surrounding the gliomas. Reactive astrocytes are universally characterized with the upregulation of glial fibrillary protein and glycoprotein podoplanin. In this work, we investigated the role of reactive astrocytes on malignant glioma microenvironment and the potential mechanism by which glioma cells activated the tumor-associated astrocytes (TAAs). The reactive astrocytes were observed around gliomas in the intracranial syngeneic implantation of rat C6 and mouse GL261 glioma cells in vivo, as well as primary astrocytes cultured with glioma cells condition medium in vitro. Besides, reactive astrocytes exhibited distinct epithelial-to-mesenchymal (-like) transition and enhanced migration and invasion activity, with the decrease of E-cadherin and concomitant increase of vimentin and matrix metalloproteinases. Furthermore, canonical Wnt/β-catenin signaling was activated in TAAs. The Wnt/β-catenin pathway inhibitor XAV939 and β-catenin plasmid were used to verify the regulation of Wnt/β-catenin signaling on TAAs and their invasion ability. Taken together, our findings established that glioma cells remarkably activated astrocytes via upregulating Wnt/β-catenin signaling, with obviously mesenchymal-like transition and increased migration and invasion ability, indicating that glioma cells may stimulate adjacent astrocytes to degrade extracellular matrix and thereby promoting tumor invasiveness.




Similar content being viewed by others
References
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507.
Placone AL, Quinones-Hinojosa A, Searson PC. The role of astrocytes in the progression of brain cancer: complicating the picture of the tumor microenvironment. Tumour Biol. 2016;37:61–9.
Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci USA. 2000;97(12):6242–4.
Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008;18(1):27–34.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–52.
Charles NA, et al. The brain tumor microenvironment. Glia. 2011;59(8):1169–80.
Pekny M, Pekna M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J Pathol. 2004;204(4):428–37.
Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35.
Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014;565:30–8.
Chen W, et al. Glioma cells escaped from cytotoxicity of temozolomide and vincristine by communicating with human astrocytes. Med Oncol. 2015;32(3):43.
Chuang HN, et al. Carcinoma cells misuse the host tissue damage response to invade the brain. Glia. 2013;61(8):1331–46.
Kolar K, et al. Podoplanin: a marker for reactive gliosis in gliomas and brain injury. J Neuropathol Exp Neurol. 2015;74(1):64–74.
Mishima K, et al. Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol. 2006;111(5):483–8.
Seike T, et al. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis. 2011;28(1):13–25.
Shabtay-Orbach A, et al. Paracrine regulation of glioma cells invasion by astrocytes is mediated by glial-derived neurotrophic factor. Int J Cancer. 2015;137(5):1012–20.
Klein A, et al. Astrocytes facilitate melanoma brain metastasis via secretion of IL-23. J Pathol. 2015;236(1):116–27.
Sin WC, et al. Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene. 2016;35:12:1504–16.
Wang L, et al. Astrocytes directly influence tumor cell invasion and metastasis in vivo. PLoS ONE. 2013;8(12):e80933.
Kim DY, Jeoung D, Ro JY. Signaling pathways in the activation of mast cells cocultured with astrocytes and colocalization of both cells in experimental allergic encephalomyelitis. J Immunol. 2010;185(1):273–83.
Kahlert UD, et al. Activation of canonical WNT/beta-catenin signaling enhances in vitro motility of glioblastoma cells by activation of ZEB1 and other activators of epithelial-to-mesenchymal transition. Cancer Lett. 2012;325(1):42–53.
Ferrer-Vaquer A, et al. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol. 2010;10:121.
Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32:121–30.
Fitzgerald DP, et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis. 2008;25(7):799–810.
Lee J, et al. Non-invasive quantification of brain tumor-induced astrogliosis. BMC Neurosci. 2011;12:9.
Ridet JL, et al. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20(12):570–7.
Shindo K, et al. Podoplanin expression in cancer-associated fibroblasts enhances tumor progression of invasive ductal carcinoma of the pancreas. Mol Cancer. 2013;12(1):168.
Astarita JL, Acton SE, Turley SJ. Podoplanin: emerging functions in development, the immune system, and cancer. Front Immunol. 2012;3:283.
Kahlert UD, Nikkhah G, Maciaczyk J. Epithelial-to-mesenchymal(-like) transition as a relevant molecular event in malignant gliomas. Cancer Lett. 2013;331(2):131–8.
Zhang K, et al. Wnt/beta-catenin signaling in glioma. J Neuroimmune Pharmacol. 2012;7(4):740–9.
Satoh J, Kuroda Y. Beta-catenin expression in human neural cell lines following exposure to cytokines and growth factors. Neuropathology. 2000;20(2):113–23.
Zhao P, et al. GSK-3beta regulates tumor growth and angiogenesis in human glioma cells. Oncotarget. 2015;6(31):31901–15.
Venning FA, Wullkopf L, Erler JT. Targeting ECM disrupts cancer progression. Front Oncol. 2015;5:224.
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos. 81372268, 81173087 and 81202611), Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. SKLNMZZCX201405), Natural Science Foundation for Distinguished Young Scholars of Jiangsu Province (No. BK20130026), the Program for Jiangsu Province Innovative Research Team, and National Found for Fostering Talents of Basic Science (No. J1030830).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Ping Lu and Yajing Wang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lu, P., Wang, Y., Liu, X. et al. Malignant gliomas induce and exploit astrocytic mesenchymal-like transition by activating canonical Wnt/β-catenin signaling. Med Oncol 33, 66 (2016). https://doi.org/10.1007/s12032-016-0778-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-016-0778-0