Abstract
Crizotinib has been reported to be particularly effective and to have acceptable toxicity in advanced anaplastic lymphoma kinase (ALK)-positive, non-small cell lung cancer (NSCLC). In this study, we analyzed the efficacy and tolerability of crizotinib in the treatment of 72 Chinese patients with ALK-positive, advanced NSCLC. All patients received oral crizotinib 250 mg twice daily in 28-day cycles during the period June 1, 2013, to October 15, 2014. The tumor response was assessed after the first cycle of crizotinib and then after every two cycles using the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0. Tolerability was assessed at least twice per cycle until crizotinib was discontinued. The patients tended to be young (mean age 55 years, range 31–83 years), never or light smokers (smoking index <400), and to have an adenocarcinoma histology. Most (49/72; 68.1 %) had received previous anticancer treatment before crizotinib therapy. Sixty-seven patients (93 %) were able to be assessed for efficacy. The objective response rate and disease control rate were 52.2 % (95 % CI 40.5–63.9 %) and 64.2 % (95 % CI 52.75–75.7 %), respectively. The estimated median progression-free survival for all 67 patients was 10.3 months (95 % CI 8.6–12.0 months). Mild visual disturbances, nausea, vomiting, diarrhea and constipation were the most commonly reported adverse effects. Thus, crizotinib was well tolerated and showed promising efficacy in Chinese patients with ALK-positive, advanced NSCLC. Further prospective, multicenter studies with a larger sample size are needed to confirm these findings.
Similar content being viewed by others
Introduction
Lung cancer, approximately 85 % of cases of which are non-small cell lung cancer (NSCLC), continues to be a leading cause of cancer deaths worldwide, posing a major threat to human health [1, 2]. Currently, individualized treatment is being emphasized for cancer patients [3], and the use of molecular-targeted therapy in genetically specific subsets of cancer patients has shown promising efficacy for many cancers, including NSCLC [4–7].
Anaplastic lymphoma kinase (ALK) is a new tyrosine kinase target that has been validated recently in NSCLC [8, 9]. ALK gene rearrangements, most often consisting of a chromosome two inversion that leads to fusion with the echinoderm microtubule-associated protein-like 4 (EML4) gene, result in abnormal expression and activation of tyrosine kinase in the cytoplasm of cancer cells [10]. In NSCLC, ALK gene rearrangements are found in only a minority of cases, occurring in 2–7 % of all NSCLC patients [8, 10, 11]. Nevertheless, several studies have indicated that ALK-positivity may represent a molecular subset of NSCLC with different clinical features, since younger patients who have an adenocarcinoma histology and have never smoked are more likely to harbor this mutation [12–16]. Recently, a large-scale study in Chinese patients showed that a younger age at diagnosis was the only independent variable associated with EML4-ALK rearrangements [17].
Recent studies with crizotinib, an oral, small-molecule, tyrosine kinase inhibitor that targets ALK, MET and ROS-1 [18–20] have demonstrated the efficacy and tolerability of this agent in advanced, ALK-positive NSCLC patients [11, 21–24]. In a phase 1, open-label, multicenter trial evaluating the efficacy and adverse event profile of crizotinib in a cohort of 82 ALK-positive lung cancer patients, treatment for a mean duration of 6.4 months achieved an overall response rate (ORR) of 57 %, and the estimated probability of 6-months’ progression-free survival (PFS) was 72 %. Mild gastrointestinal disturbances were the main adverse effects observed in this study [11]. Subsequently, updated data from a study involving 143 patients confirmed the durable response and tolerable adverse effect profile of crizotinib in patients with ALK-positive NSCLC [24]. More recently, a phase 3, open-label study comparing crizotinib with standard chemotherapy indicated that crizotinib was superior to pemetrexed or docetaxel in previously treated, ALK-positive, advanced NSCLC. Response rates were 65 % with crizotinib as compared with 20 % with the standard chemotherapy regimens (p < 0.001) [22].
In the present study, we analyzed the efficacy and tolerability of crizotinib in the treatment of advanced, ALK-positive NSCLC in Chinese patients.
Patients and methods
Patients
A total of 72 patients with ALK-positive NSCLC who received crizotinib between June 1, 2013, and October 15, 2014, at Shanghai Chest Hospital, Shanghai Jiao Tong University, were prospectively enrolled in the study. All were histologically diagnosed and staged as clinically advanced (stage IV or stage IIIB with pleural effusion) NSCLC. Before initiation of therapy, all patients were evaluated by computed tomography (CT) of the thorax, an electrocardiogram (ECG), enhanced magnetic resonance imaging (MRI) of the brain and abdominal ultrasound. In addition, routine hematology tests (e.g., red blood cell counts, hemoglobin, white blood cell and platelet counts), biochemistry analyses (including alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, glutamyl endopeptidase and lactate dehydrogenase), coagulation tests and urinalyses were performed, and a medical history was taken from each patient. Patients with symptomatic brain metastases or an Eastern Cooperative Oncology Group performance status (ECOG PS) of more than two were not enrolled. There was no limit to the number of previous treatment regimens for any patient. Age, gender, smoking status, histologic type, epidermal growth factor receptor (EGFR) mutation status, clinical stage, number of previous therapies, the overall response, PFS and toxicity were all recorded.
The study was approved by Ethics Committee of Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai. All patients provided written informed consent before enrollment.
Crizotinib treatment and response evaluation
All patients received oral crizotinib 250 mg twice daily in 28-day cycles. The tumor response was assessed after the first cycle of crizotinib therapy and subsequently after every two cycles using the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0 [25]. CT scans were used to assess the response to crizotinib as clinically indicated or until discontinuation of treatment. The overall response to crizotinib was reported as either a complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). Patients continued to receive crizotinib treatment as long as they did not have PD or intolerable adverse effects. The cutoff date for the study was December 1, 2014.
Molecular pathology detection
For EGFR and ALK mutation detection, tumor samples were obtained via either diagnostic or surgical procedures. We used the ADx EGFR Mutation Detection Kit (Amoy Diagnostics, Xiamen, China), which has received approval by China’s Food and Drug Administration (CFDA) for clinical usage in mainland China. The principle of amplification refractory mutation system (ARMS) was used in the kit. Fluorescence in situ hybridization (FISH) and immunohistochemical methods were used for ALK detection.
Immunohistochemical analysis was conducted with the monoclonal antibody D5F3 (Ventana Medical Systems, Tucson, AZ, USA).
Assessment of tolerability
Tolerability was assessed at least twice in each treatment cycle until crizotinib was discontinued by the occurrence of adverse events, ECGs, routine hematology and biochemistry testing, coagulation tests and urinalyses. All toxicities were summarized and reported according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE), version 3.0.
Statistical analysis
Pearson’s χ 2 tests and Fisher’s exact tests were used for analyzing the ORR and disease control rate (DCR) according to patient characteristics. PFS was defined as the time from the date crizotinib was first administered until the date of objective PD or the death of any patient. The Kaplan–Meier method was used to estimate PFS, and log-rank tests were used to compare differences between subgroups. All confidence intervals reported were two-sided, and p values <0.05 were considered statistically significant. Statistical analysis was performed using SPSS® software, version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
The demographic and clinicopathologic characteristics of the 72 ALK-positive NSCLC patients enrolled in the study are summarized in Table 1. Patients tended to be young (mean age 55 years, range 31–83 years), never or light smokers (smoking index <400), with an adenocarcinoma histology. Most patients (49/72; 68.1 %) had received previous anticancer treatment prior to crizotinib therapy. Of those with a known EGFR mutation status (n = 39), one patient (2.6 %) was found to have a leucine-to-arginine substitution at position 858 (21L858R).
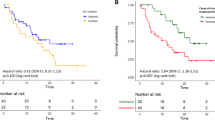
Five patients (6.9 %) discontinued crizotinib therapy at an early stage due to either intolerable adverse effects or other reasons, and these patients were not included in the subsequent efficacy analysis, although they were included in the safety evaluation. At the study cutoff date, the median duration of treatment for the 67 patients who were evaluable for efficacy was 5.7 months (range 1.0–17.7 months); 47 patients (70.1 %) continued to receive crizotinib therapy until the cutoff date (Fig. 1).
Efficacy evaluation
Of the 67 patients evaluable for efficacy, one (1.5 %) met the RECIST criteria for a CR and 34 (50.8 %) were confirmed as having a PR. Twenty-four patients (35.8 %) had PD at the cutoff date, while eight (11.9 %) were evaluated as SD. The ORR (CR and PR) was 52.2 % (95 % CI 40.5–63.9 %), and the DCR (CR, PR and SD) was 64.2 % (95 % CI 52.7–75.7 %). On the basis of patient characteristics, the proportion of patients with an objective response rate and those with the DCR were similar regardless of age (<60 vs. ≥60 years), radical surgery history, histologic type and EGFR mutation status. The ORR and DCR were lower among those who had received multiple-line (third-line or later) treatment prior to crizotinib therapy, but the differences were not statistically significant (p = 0.702 and p = 0.588, respectively; Table 2). Although the ORR of female patients was significantly higher than that of males (64.7 vs. 39.4 %, respectively; p = 0.038), this was not the case for the DCR (p = 0.548; Table 2). Four of the 24 patients (16.7 %) with disease progression continued to receive crizotinib, two of whom were given sequential crizotinib and standard chemotherapy as they were deriving ongoing clinical benefit from crizotinib.
The estimated median PFS for all 67 patients was 10.3 months (95 % CI 8.6–12.0 months; Fig. 2). In patients receiving first-line crizotinib therapy (n = 21), the median PFS was 14.3 months (95 % CI 9.4–19.1 months; Fig. 3a), while in patients receiving crizotinib as second-line or later treatment (n = 46), the median PFS was 10.0 months (95 % CI 7.2–13.0 months; Fig. 3a). However, there was no statistically significant difference between these two groups (p = 0.352). Similar results were found when comparing PFS between second-line and other-line treatment, and multiple-line and other-line treatment (p = 0.932 and p = 0.257, respectively; Fig. 3b, c). At the study cutoff date, the median overall survival (OS) had not been reached; 18 of the 67 patients (26.9 %) had died, and 49 (73.1 %) were still being followed up for survival. The estimated OS at 6 and 12 months was 85.1 % (95 % CI 76.6–93.6 %) and 77.6 % (95 % CI 67, 0.6–87.7 %), respectively.
a Kaplan–Meier curves of progression-free survival for crizotinib as first-line treatment and other-line treatment (p = 0.352, log-rank test). b Kaplan–Meier curves of progression-free survival for crizotinib as second-line treatment and other-line treatment (p = 0.932, log-rank test). c Kaplan–Meier curves of progression-free survival for crizotinib as third-line treatment and other-line treatment (p = 0.257, log-rank test). Tick marks represent censored observations
Tolerability
Sixty-four of the 72 treated patients (89 %) experienced crizotinib-related adverse events (Table 3). Mild visual disturbances were the most common adverse effects and were reported by 35 patients (49 %). As well as visual disturbances, gastrointestinal disturbances, including nausea, vomiting, diarrhea and constipation, were also frequent treatment-related adverse effects. In addition, decreased appetite, elevations in alanine aminotransferase (ALT) and aspartate aminotransferase (AST), peripheral edema, fatigue, dizziness and rash were also reported commonly. However, most of these reactions were transient during crizotinib treatment.
Grade 3 or 4 adverse events were observed in ten patients (14 %). Grade 3 elevations in ALT and AST were observed in three (4 %) and two patients (3 %), respectively, while five patients experienced grade 3 vomiting and one patient had grade 3 pneumonitis. In one patient, an acute pulmonary embolism occurred less than a month after beginning crizotinib therapy, which led to death. Four patients (6 %) discontinued crizotinib (three for vomiting and one for elevations of ALT and AST), while two patients (3 %) required a dosage reduction because of treatment-related adverse events (one for vomiting and one for elevations of ALT and AST).
Discussion
The findings of this study suggest that crizotinib is well tolerated and has promising efficacy in ALK-positive, advanced NSCLC in Chinese patients, with an ORR of more than 50 % and a median PFS of more than 10 months.
As previously reported [12–15], we found that patients who were ALK-positive were predominantly young, never or light smokers, and had an adenocarcinoma histology. Of those with a known EGFR mutation status, one ALK-positive patient had a leucine-to-arginine substitution at position 858 (21L858R). The incidence of ALK rearrangement and concurrent EGFR mutations may be lower than previously reported, and a possible reason for this might be that more EGFR mutations occur among the status-unknown population. EGFR mutation patients share some crucial clinical characteristics with patients with ALK rearrangement, such as an adenocarcinoma histology and never or light smoking. However, a recent large-scale study in Chinese people showed that a younger age at diagnosis was the only independent variable associated with EML4-ALK rearrangements, while lower tobacco exposure, adenocarcinoma histology and moderate-to-high tumor differentiation were independently associated with EGFR mutations [17]. ALK rearrangements typically occur independently of EGFR and KRAS gene mutations [12, 13, 16, 26]. Thus, EGFR mutations may define a specific molecular subgroup of NSCLC.
Crizotinib showed encouraging efficacy in the present study with more than half of the patients achieving an objective response. The ORR was largely independent of age, performance status, line of therapy, radical surgery history, histologic type, clinical stage, smoking status or EGFR mutation status, but female patients seemed to obtain a better objective response rate than males. However, the DCR, which reached 64.2 % in this study, seemed largely independent of all of the above characteristics.
The median PFS in our study population was over 10 months. This is higher than in previous studies, and a possible reason for this may be that crizotinib exposure in Asian patients is higher than in other populations. We found no statistically significant differences in PFS between different lines of therapy. OS data had not been reached at the study cutoff date, and most patients were still being followed up for survival. The estimated OS at 6 and 12 months was 85.1 and 77.6 %, respectively. In a previous study that assessed the efficacy of crizotinib in ALK-positive patients [23], the median OS was not reached (95 % CI 17 months to not reached), indicating that ALK-positive patients may achieve a long survival time with this agent. In addition, this study indicated that OS did not differ based on age, sex, smoking history or ethnic origin [23].
Crizotinib seemed to be well tolerated in our study, although the majority of cases experienced treatment-related adverse events. However, most adverse events were mild and transient. Mild visual disturbances were the most commonly reported adverse events, with about half of patients experiencing this toxicity. Grade 3 or 4 gastrointestinal reactions and liver dysfunction were the main reasons for crizotinib dosage reductions or discontinuation. Notably, one patient suffered an acute pulmonary embolism which led to death less than a month after beginning crizotinib treatment.
Four patients with PD in our study continued to receive crizotinib as we believed these patients would acquire clinical benefit from the drug, despite being assessed as PD by RECIST. However, our OS data were not mature. Recently, a retrospective analysis indicated that continuing ALK inhibition with crizotinib after PD may provide a survival benefit to patients with advanced, ALK-positive NSCLC [27]. RECIST-defined PD can be used as an indication for chemotherapy withdrawal, but not always for the withdrawal of targeted therapies [28]. Some of the limitations of RECIST are cancer and therapy specific and are observed in patients with specific genomic mutations treated with specific targeted therapies [29, 30]. Thus, whether long-term clinical benefit is derived from crizotinib will need to be reassessed for patients with RECIST-defined PD.
The methods to detect ALK rearrangement are not yet uniform. Current approaches to detecting ALK rearrangement mainly include immunochemistry, reverse transcription polymerase chain reaction (RT-PCR) technology and FISH. All three methodologies have shown good sensitivity, specificity and concordance [31–33]. FISH analysis is considered the gold standard for the detection of ALK rearrangement. However, this method is relatively time-consuming and expensive, and its routine application is difficult. Immunochemistry for the detection of ALK rearrangement can be used for limited tissue samples, and this technique has simple and feasible procedures [34]. Some studies have suggested that immunochemistry can be used as a tool for large-scale screening, but the results need to be confirmed by other methods [33, 35, 36]. In this study, we used monoclonal antibody D5F3, which showed the best sensitivity among three clones (ALK1 clone, 5A4 clone and D5F3 clone) and a specificity of 100 % [31, 32] to screen for ALK rearrangement, which was then verified by FISH.
Our study has some limitations, including the fact that it was a single-center study with a relatively small sample size. Consequently, its findings need to be confirmed by subsequent multicenter studies with larger sample sizes. In addition, when considering the efficacy of crizotinib, the study’s results should be interpreted with caution since most patients had previously received systemic anticancer treatment before receiving crizotinib. Such treatments may, in some way, affect the efficacy of crizotinib. A previous study [24] and our findings suggest that the response rate is largely independent of the line of treatment [24]. We found no statistically significant differences in PFS between different lines of treatment.
In conclusion, crizotinib was well tolerated and showed promising efficacy in Chinese patients with ALK-positive, advanced NSCLC. To the best of our knowledge, this is the first study assessing the efficacy and tolerability of crizotinib in the treatment of ALK-positive, advanced NSCLC in Chinese subjects. However, due to the limitations of this work, prospective multicenter analysis with a larger sample size is needed to confirm these findings.
References
Shibuya K, Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJ. Global and regional estimates of cancer mortality and incidence by site. II. Results for the global burden of disease 2000. BMC Cancer. 2002;2:36.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Jackson SE, Chester JD (2014) Personalised cancer medicine. Int J Cancer May 2, 2014 [Epub ahead of print].
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80.
Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7.
Fan Y, Xu X, Xie C. EGFR-TKI therapy for patients with brain metastases from non-small-cell lung cancer: a pooled analysis of published data. Onco Targets Ther. 2014;7:2075–84.
Metro G, Finocchiaro G, Toschi L, Bartolini S, Magrini E, Cancellieri A, Trisolini R, Castaldini L, Tallini G, Crino L, Cappuzzo F. Epidermal growth factor receptor (EGFR) targeted therapies in non-small cell lung cancer (NSCLC). Rev Recent Clin Trials. 2006;1:1–13.
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6.
Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–203.
Gridelli C, Peters S, Sgambato A, Casaluce F, Adjei AA, Ciardiello F. ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat Rev. 2014;40:300–6.
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, Iafrate AJ. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703.
Takahashi T, Sonobe M, Kobayashi M, Yoshizawa A, Menju T, Nakayama E, Mino N, Iwakiri S, Sato K, Miyahara R, Okubo K, Manabe T, Date H. Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 2010;17:889–97.
Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S, McDermott U, Settleman J, Kobayashi S, Mark EJ, Rodig SJ, Chirieac LR, Kwak EL, Lynch TJ, Iafrate AJ. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–53.
Fukui T, Yatabe Y, Kobayashi Y, Tomizawa K, Ito S, Hatooka S, Matsuo K, Mitsudomi T. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer. 2012;77:319–25.
Wang Y, Wang S, Xu S, Qu J, Liu B. Clinicopathologic features of patients with non-small cell lung cancer harboring the EML4-ALK fusion gene: a meta-analysis. PLoS One. 2014;9:e110617.
Wang J, Dong Y, Cai Y, Zhou L, Wu S, Liu G, Su D, Li X, Qin N, Nong J, Jia H, Zhang Q, Mu J, Zeng X, Zhang H, Zhang S, Zhang Z. Clinicopathologic characteristics of ALK rearrangements in primary lung adenocarcinoma with identified EGFR and KRAS status. J Cancer Res Clin Oncol. 2014;140:453–60.
Hong S, Fang W, Hu Z, Zhou T, Yan Y, Qin T, Tang Y, Ma Y, Zhao Y, Xue C, Huang Y, Zhao H, Zhang L. A large-scale cross-sectional study of ALK rearrangements and EGFR mutations in non-small-cell lung cancer in Chinese Han population. Sci Rep. 2014;4:7268.
Frampton JE. Crizotinib: a review of its use in the treatment of anaplastic lymphoma kinase-positive, advanced non-small cell lung cancer. Drugs. 2013;73:2031–51.
Ou SH, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW, Camidge DR, Solomon BJ, Maki RG, Bang YJ, Kim DW, Christensen J, Tan W, Wilner KD, Salgia R, Iafrate AJ. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6:942–6.
Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang R, Mark EJ, Batten JM, Chen H, Wilner KD, Kwak EL, Clark JW, Carbone DP, Ji H, Engelman JA, Mino-Kenudson M, Pao W, Iafrate AJ. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–70.
Qian H, Gao F, Wang H, Ma F. The efficacy and safety of crizotinib in the treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer: a meta-analysis of clinical trials. BMC Cancer. 2014;14:683.
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, Wu YL, Thomas M, O’Byrne KJ, Moro-Sibilot D, Camidge DR, Mok T, Hirsh V, Riely GJ, Iyer S, Tassell V, Polli A, Wilner KD, Jänne PA. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94.
Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, Shapiro GI, Costa DB, Ou SH, Butaney M, Salgia R, Maki RG, Varella-Garcia M, Doebele RC, Bang YJ, Kulig K, Selaru P, Tang Y, Wilner KD, Kwak EL, Clark JW, Iafrate AJ, Camidge DR. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–12.
Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, Riely GJ, Solomon B, Ou SH, Kim DW, Salgia R, Fidias P, Engelman JA, Gandhi L, Jänne PA, Costa DB, Shapiro GI, Lorusso P, Ruffner K, Stephenson P, Tang Y, Wilner K, Clark JW, Shaw AT. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–9.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Zhang X, Zhang S, Yang X, Yang J, Zhou Q, Yin L, An S, Lin J, Chen S, Xie Z, Zhu M, Zhang X, Wu YL. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer. 2010;9:188.
Ou SH, Janne PA, Bartlett CH, Tang Y, Kim DW, Otterson GA, Crinò L, Selaru P, Cohen DP, Clark JW, Riely GJ. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol. 2014;25:415–22.
Nishino M, Jagannathan JP, Krajewski KM, O’Regan K, Hatabu H, Shapiro G, Ramaiya NH. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198:737–45.
Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, Macapinlac HA, Podoloff DA. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004;183:1619–28.
Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–9.
Demidova I, Barinov A, Savelov N, Gagarin I, Grinevitch V, Stroiakovaski D, Popov M, Laktionov K, Gutorov S, Smolin A, Olshanskaya Y, Obukhova T. Immunohistochemistry, fluorescence in situ hybridization, and reverse transcription-polymerase chain reaction for the detection of anaplastic lymphoma kinase gene rearrangements in patients with non-small cell lung cancer: potential advantages and methodologic pitfalls. Arch Pathol Lab Med. 2014;138:794–802.
Selinger CI, Rogers TM, Russell PA, O’Toole S, Yip P, Wright GM, Wainer Z, Horvath LG, Boyer M, McCaughan B, Kohonen-Corish MR, Fox S, Cooper WA, Solomon B. Testing for ALK rearrangement in lung adenocarcinoma: a multicenter comparison of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol. 2013;26:1545–53.
Martinez P, Hernández-Losa J, Montero MÁ, Cedrés S, Castellví J, Martinez-Marti A, Tallada N, Murtra-Garrell N, Navarro-Mendivill A, Rodriguez-Freixinos V, Canela M, Ramon y Cajal S, Felip E. Fluorescence in situ hybridization and immunohistochemistry as diagnostic methods for ALK positive non-small cell lung cancer patients. PLoS One. 2013;8(1):e52261.
Akiba J, Kawahara A, Abe H, Azuma K, Yamaguchi T, Taira T, Fukumitsu C, Takase Y, Yasumoto M, Umeno Y, Todoroki K, Kurita T, Yamaguchi R, Kage M, Yano H. Evaluation of immunohistochemistry using two different antibodies and procedures for primary lung adenocarcinoma harboring anaplastic lymphoma kinase rearrangement. Oncol Lett. 2014;8:2155–9.
Ali G, Proietti A, Pelliccioni S, Niccoli C, Lupi C, Sensi E, Giannini R, Borrelli N, Menghi M, Chella A, Ribechini A, Cappuzzo F, Melfi F, Lucchi M, Mussi A, Fontanini G. ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med. 2014;138:1449–58.
Wang J, Cai Y, Dong Y, Nong J, Zhou L, Liu G, Su D, Li X, Wu S, Chen X, Qin N, Zeng X, Zhang H, Zhang Z, Zhang S. Clinical characteristics and outcomes of patients with primary lung adenocarcinoma harboring ALK rearrangements detected by FISH, IHC, and RT-PCR. PLoS One. 2014;9:e101551.
Acknowledgments
Editorial assistance with the manuscript was provided by Content Ed Net, Shanghai Co. Ltd.
Conflict of interest
None of the authors has any conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, S., Zhao, Y., Gu, A. et al. Efficacy and tolerability of crizotinib in the treatment of ALK-positive, advanced non-small cell lung cancer in Chinese patients. Med Oncol 32, 180 (2015). https://doi.org/10.1007/s12032-015-0626-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0626-7