Abstract
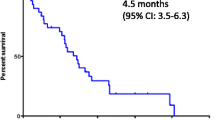
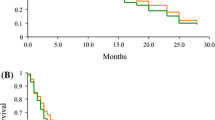
The aim of this clinical study was to evaluate the efficacy and safety of nab-paclitaxel to treat patients with advanced non-small cell lung cancer (NSCLC) who failed first-line chemotherapy. Eligible patients had advanced NSCLC and had been treated with first-line platinum-based chemotherapy but still had disease progression. Patients received nab-paclitaxel 100 mg/m2 (i.v.) on days 1, 8 and 15 of a 28-day cycle. Primary endpoint is 6-month progression-free survival (PFS). Fifty-six patients with advanced NSCLC were enrolled in the study (55.4 % male patients, 44.6 % female patients; median age 59.6 years; ranging from 32 to 83 years). Six-month PFS rate was 18 % (95 % CI 7.8–28.7 %). Median PFS was 3.5 months (95 % CI 1.9–5.8 months). Median overall survival was 6.8 months (95 % CI 4.7–9.3 months). No complete responses were achieved. Overall response rate was 16.1 % (95 % CI 8.9–24.7 %). Grade 3 or 4 adverse events (AEs) were observed in patients receiving nab-paclitaxel. The most common grade 3 or 4 AEs were dizziness, pulmonary embolism and fatigue. Nab-paclitaxel showed clinically equivalent efficacy on patients’ survivals and response rates, as compared with other FDA-approved second-line chemotherapy agents. Given the tolerability on grade 3 or 4 adverse events, nab-paclitaxel may be considered an alternative second-line treatment option for NSCLC.

Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
Walker S. Updates in non-small cell lung cancer. Clin J Oncol Nurs. 2008;12(4):587–96.
Al-Farsi A, Ellis PM. Treatment paradigms for patients with metastatic non-small cell lung cancer, squamous lung cancer: first, second, and third-line. Front oncol. 2014;4:157.
Group NM-aC. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383(9928):1561–71.
Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, The TAX 320 NON-Small Cell Lung Cancer Study Group, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol. 2000;18(12):2354–62.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–32.
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clinical Oncol. 2004;22(9):1589–97.
Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P, Zhang H, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–62.
Socinski MA, Okamoto I, Hon JK, Hirsh V, Dakhil SR, Page RD, Orsini J, Yamamoto N, Zhang H, Renschler MF. Safety and efficacy analysis by histology of weekly nab-paclitaxel in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24(9):2390–6.
Fang Y, Wang L, Xia GH, Shi MQ. Clinical investigation of efficacy of albumin bound paclitaxel plus platinum compounds as first-line chemotherapy for stage III/IV squamous non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15(17):7453–7.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Hosein PJ, de Lima Lopes G, Pastorini VH, Gomez C, Macintyre J, Zayas G, Reis I, Montero AJ, Merchan JR, Rocha Lima CM. A phase II trial of nab-paclitaxel as second-line therapy in patients with advanced pancreatic cancer. Am J Clin Oncol. 2013;36(2):151–6.
Ko YJ, Canil CM, Mukherjee SD, Winquist E, Elser C, Eisen A, Reaume MN, Zhang L, Sridhar SS. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study. Lancet Oncol. 2013;14(8):769–76.
Sasaki Y, Nishina T, Yasui H, Goto M, Muro K, Tsuji A, Koizumi W, Toh Y, Hara T, Miyata Y. Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci. 2014;105(7):812–7.
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN. CTCAE v3. 0: development of a comprehensive grading system for the adverse effects of cancer treatment. In: Seminars in radiation oncology. Elsevier; 2003.p. 176–181.
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18(10):2095–103.
Massarelli E, Andre F, Liu DD, Lee JJ, Wolf M, Fandi A, Ochs J, Le Chevalier T, Fossella F, Herbst RS. A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer. 2003;39(1):55–61.
Spigel DR, Burris HA 3rd, Greco FA, Shipley DL, Friedman EK, Waterhouse DM, Whorf RC, Mitchell RB, Daniel DB, Zangmeister J, et al. Randomized, double-blind, placebo-controlled, phase II trial of sorafenib and erlotinib or erlotinib alone in previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(18):2582–9.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, W., Zhang, Z. A phase II clinical study of using nab-paclitaxel as second-line chemotherapy for Chinese patients with advanced non-small cell lung cancer. Med Oncol 32, 177 (2015). https://doi.org/10.1007/s12032-015-0498-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0498-x