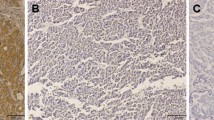
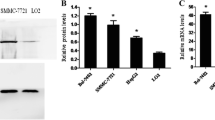
Abstract
VEGFR-1-mediated signaling promotes invasiveness by direct tumor activation in some cancers. However, VEGFR-1 expression and its relationship with clinical features and prognosis in HCC remained unclear. Overexpression of Snail is common in HCC and associated with poorer prognosis. Therefore, expression of VEGFR-1 and Snail was investigated in HCC cell lines and tissue specimens in our study, and special attention was paid to evaluating the role of VEGFR-1 expression in prognosis of HCC. Western blot and immunofluorescence were used to detect expression of VEGFR-1, Snail and MMP-9 in 4 HCC cell lines, respectively. Moreover, expression of these proteins was confirmed on the samples from 95 HCC patients who underwent curative resection using immunohistochemistry. ROC and survival analysis determined the predictive values of parameters and the association with survival. HCC cell lines with a higher VEGFR-1 expression were more invasive. Both VEGFR-1 and Snail expressions were significantly higher in HCC tissues than in non-cancerous tissues. High-expression VEGFR-1 and Snail, associated with adverse clinical features, were independent prognostic factors for RFS and OS (P = 0.023 and P = 0.044, respectively). Positive correlation was found between VEGFR-1 and Snail or MMP-9 (r = 0.418 and r = 0.232, respectively, P < 0.05 for both) in cancerous tissues. The combination of VEGFR-1 and Snail gave a better power to predict patients’ recurrence and death (P < 0.001 for both). High VEGFR-1 expression, distinctively expressed in the cytomembrane of HCC cells, was associated with HCC progression and worse outcome. High co-expression of VEGFR-1 and Snail may be a novel prognostic marker for HCC, especially in recurrence.






Similar content being viewed by others
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- VEGFR-1:
-
Vascular endothelial growth factor receptor-1
- TNM:
-
Tumor-node metastasis
- MMP-9:
-
Matrix metalloproteinase-9
- TMA:
-
Tissue microarray
- ROC:
-
Receiver operating characteristic
- AFP:
-
α-fetoprotein
- EMT:
-
Epithelial–mesenchymal transition
- HBsAg:
-
Hepatitis B s antigen
- OS:
-
Overall survival
- RFS:
-
Recurrence-free survival
References
Tang ZY, Ye SL, Liu YK, et al. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–96.
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17.
Hao K, Luk JM, Lee NP, et al. Predicting prognosis in hepatocellular carcinoma after curative surgery with common clinicopathologic parameters. BMC Cancer. 2009;3:389.
Qin LX, Tang ZY. Recent progression in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol. 2004;130:497–513.
Fong GH, Klingensmith J, Wood CR, et al. Regulation of Flt-1 expression during mouse embryogenesis suggests a role in the establishment of vascular endothelium. Dev Dyn. 1996;207:1–10.
Kearney JB, Kappas NC, Ellerstrom C, et al. The VEGF receptor flt-1(VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–35.
Wey JS, Fan F, Gray MJ, et al. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer. 2005;104:427–38.
Yang AD, Camp ER, Fan F, et al. Vascular endothelial growth factor receptor-1 activation mediates epithelial to Mesenchymal transition in human pancreatic carcinoma cells. Cancer Res. 2006;66:46–51.
Fan F, Wey JS, McCarty MF, et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24:2647–53.
Bates RC, Goldsmith JD, Bachelder RE, et al. Flt-1 dependent survival characterizes the epithelial-mesenchymal transition of colonic organoids. Curr Biol. 2003;13:1721–7.
Wu Y, Hooper AT, Zhong Z, et al. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119:1519–29.
Yoshiji H, Kuriyama S. ea al. Involvement of the vascular endothelial growth factor receptor-1 in murine hepatocellular carcinoma development. J Hepatol. 2004;41:97–103.
Dales JP, Garcia S, Bonnier P, et al. Prognostic significance of VEGF receptors, VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1) in breast carcinoma. Ann Pathol. 2003;23:297–305.
Mylona E, Alexandrou P, Giannopoulou I, et al. The prognostic value of vascular endothelial growth factors (VEGFs)-A and -B and their receptor, VEGFR-1, in invasive breast carcinoma. Gynecol Oncol. 2007;104:557–63.
Kanda M, Nomoto S, Nishikawa Y, et al. Correlation of the expression of vascular endothelial growth factor B and its isoforms in hepatocellular carcinoma with clinico-pathological parameters. J Surg Oncol. 2008;98:190–6.
Grau Y, Carteret C, Simpson P. Mutations and chromosomal rearrangements affecting the expression of snail, a gene involved in embryonic patterning in Drosophila melanogaster. Genetics. 1984;108:347–60.
Leptin M, Grunewald B. Cell shape changes during gastrulation in Drosophila. Development. 1990;110:73–84.
Jiang J, Tang Y, Zhu G, et al. Correlation between transcription factor Snail expression and prognosis in adenoid cystic carcinoma of salivary gland. Oral Surg Oral Med Oral Pathol Oral Radiol. 2010;110:764–9.
Kosaka T, Kikuchi E, Mikami S, et al. Expression of snail in upper urinary tract urothelial carcinoma: prognostic significance and implications for tumor invasion. Clin Cancer Res. 2010;16:5814–23.
Blanco MJ, Moreno-Bueno G, Sarrio D, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinoma. Oncogene. 2002;21:3241–6.
Häyry V, Salmenkivi K, Arola J, et al. High frequency of SNAIL-expressing cells confirms and predicts metastatic potential of phaeochromocytoma. Endocr Relat Cancer. 2009;16:1211–8.
Sugimachi K, Tanaka S, Kameyama T, et al. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin Cancer Res. 2003;9:2657–64.
Miyoshi A, Kitajima Y, Kido S, et al. Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. Br J Cancer. 2005;92:252–8.
Jiao W, Miyazaki K, Kitajima Y. Inverse correlation between E-cadherin and Snail expression in hepatocellular carcinoma cell lines in vitro and in vivo. Br J Cancer. 2002;86:98–101.
Sun L, Diamond ME, Ottaviano AJ, et al. Transforming growth factor-beta 1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through snail expression. Mol Cancer Res. 2008;6:10–20.
Wanami LS, Chen HY, Peiró S, et al. Vascular endothelial growth factor-A stimulates Snail expression in breast tumor cells: implications for tumor progression. Exp Cell Res. 2008;314:2448–53.
Yamamoto H, Itoh F, Adachi Y, et al. Relation of enhanced secretion of active matrix metalloproteinases with tumor spread in human hepatocellular carcinoma. Gastroenterology. 1997;112:1290–6.
Bloomston M, Zervos EE, Rosemurgy AS II. Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann Surg Oncol. 2002;9:668–74.
Chu D, Zhao Z, Zhou Y, et al. Matrix metalloproteinase-9 is associated with relapse and prognosis of patients with colorectal cancer. Ann Surg Oncol. 2011;. doi:10.1245/s10434-011-1836-7.
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74.
Jordà M, Olmeda D, Vinyals A, et al. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J Cell Sci. 2005;118:3371–85.
Hiratsuka S, Nakamura K, Iwai S, et al. MMP-9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300.
Zhang S, Li J, Jiang Y, et al. Programmed cell death 4 (PDCD4) suppresses metastatic potential of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2009;28:71.
Li N, Yi F, Sundy CM, et al. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol. 2007;292:F207–16.
Sun HC, Tang ZY, Li XM, et al. Microvessel density of hepatocellular carcinoma: its relationship with prognosis. J Cancer Res Clin Oncol. 1999;125:419–26.
Livraghi T. Radiofrequency ablation, PEIT, and TACE for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2003;10:67–76.
Shen SQ, Li K, Zhu N, Nakao A. Expression and clinical significance of NET-1 and PCNA in hepatocellular carcinoma. Med Oncol. 2008;25:341–5.
Jia JB, Zhuang PY, Sun HC, et al. Protein expression profiling of vascular endothelial growth factor and its receptors identifies subclasses of hepatocellular carcinoma and predicts survival. J Cancer Res Clin Oncol. 2009;135:847–54.
McCarty KS Jr, Szabo E, Flowers JL, et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986;46:4244s–8s.
Toi M, Bando H, Ogawa T, et al. Significance of vascular endothelial growth factor (VEGF)/soluble VEGF receptor-1 relationship in breast cancer. Int J Cancer. 2002;98:14–8.
Ng IO, Poon RT, Lee JM, et al. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol. 2001;116:838–45.
Amaoka N, Saio M, Nonaka K, et al. Expression of vascular endothelial growth factor receptors is closely related to the histological grade of hepatocellular carcinoma. Oncol Rep. 2006;16:3–10.
González-Cuevas J, Bueno-Topete M, Armendariz-Borunda J. Urokinase plasminogen activator stimulates function of active forms of stromelysin and gelatinases (MMP-2 and MMP-9) in cirrhotic tissue. J Gastroenterol Hepatol. 2006;21:1544–54.
Miura K, Yoshino R, Hirai Y, et al. Epimorphin, a morphogenic protein, induces proteases in rodent hepatocytes through NF-kappaB. J Hepatol. 2007;47:834–43.
Fukase K, Ohtsuka H, Onogawa T, et al. Bile acids repress E-cadherin through the induction of Snail and increase cancer invasiveness in human hepatobiliary carcinoma. Cancer Sci. 2008;99:1785–92.
Miyoshi A, Kitajima Y, Sumi K, et al. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer. 2004;90:1265–73.
Acknowledgments
We are grateful to Dr. Edward C. Mignot for professional linguistic advice. We are also grateful to Professor Ling Gao (Central Laboratory of Provincial Hospital Affiliated to Shandong University, Jinan, China) for helpful discussion regarding this research area. This study was supported by Shandong Outstanding Youth Science Fund (No. 2007BS03005).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, T., Zhu, Y., Ren, W. et al. High co-expression of vascular endothelial growth factor receptor-1 and Snail is associated with poor prognosis after curative resection of hepatocellular carcinoma. Med Oncol 29, 2750–2761 (2012). https://doi.org/10.1007/s12032-012-0160-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-012-0160-9