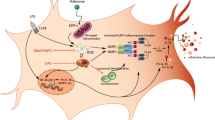
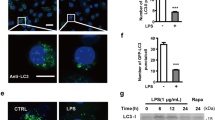
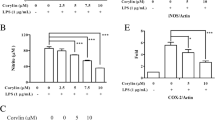
Abstract
The activation of microglia is an important cause of central nervous system (CNS) inflammatory cell infiltration and inflammatory demyelination in experimental autoimmune encephalomyelitis (EAE). Furthermore, the proinflammatory response induced by the NLR family pyrin domain containing 3 (NLRP3) inflammasome can be amplified in microglia after NLRP3 inflammasome activation. Autophagy is closely related to the inflammatory response. Caffeine exerts anti-inflammatory and autophagy-stimulating effects, but the specific mechanism remains unclear. This study examined the mechanism underlying the anti-inflammatory effect of caffeine on EAE. In this study, C57BL/6 mice were immunized to induce EAE and treated with caffeine to observe its effect on prognosis. The effects of caffeine on autophagy and inflammation were also analysed in mouse primary microglia (PM) and the BV2 cell line. The data demonstrated that caffeine reduced the clinical score, the infiltration of inflammatory cells, the demyelination level, and the activation of microglia in EAE mice. Furthermore, caffeine increased the LC3-II/LC3-I levels and decreased the NLRP3 and P62 levels in EAE mice, whereas the autophagy inhibitor 3-methylamine (3-MA) blocked these effects. In vitro, caffeine promoted autophagy by suppressing the mechanistic target of rapamycin (mTOR) pathway and inhibited activation of the NLRP3 inflammasome. However, autophagy-related gene 5 (ATG5)-specific siRNA abolished the anti-inflammatory effect of caffeine treatment in PM and BV2 cells. Taken together, these data suggest that caffeine exerts a newly discovered effect on EAE by reducing NLRP3 inflammasome activation via the induction of autophagy in microglia.






Similar content being viewed by others
Availability of Data and Materials
The data in this study are available upon reasonable request.
References
Antonioli M, Di Rienzo M, Piacentini M, Fimia GM (2017) Emerging Mechanisms in Initiating and Terminating Autophagy. Trends Biochem Sci 42(1):28–41. https://doi.org/10.1016/j.tibs.2016.09.008
Ballabio A, Bonifacino JS (2020) Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat Rev Mol Cell Biol 21(2):101–118. https://doi.org/10.1038/s41580-019-0185-4
Barclay W, Shinohara ML (2017) Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Brain Pathol 27(2):213–219. https://doi.org/10.1111/bpa.12477
Berglund R, Guerreiro Cacais AO, Adzemovic MZ, Zeitelhofer M, Lund H, Ewing E, Ruhrmann S, Nutma E, Parsa R, Thessen Hedreul M, Amor S, Harris RA, Olsson T, Jagodic M (2020) Microglial autophagy-associated phagocytosis is essential for recovery from neuroinflammation. Sci Immunol 5(52). https://doi.org/10.1126/sciimmunol.abb5077
Boia R, Elvas F, Madeira MH, Aires ID, Rodrigues-Neves AC, Tralhão P, Szabó EC, Baqi Y, Müller CE, Tomé ÂR, Cunha RA, Ambrósio AF, Santiago AR (2017) Treatment with A receptor antagonist KW6002 and caffeine intake regulate microglia reactivity and protect retina against transient ischemic damage. Cell Death Dis 8(10):e3065. https://doi.org/10.1038/cddis.2017.451
Briard B, Fontaine T, Samir P, Place DE, Muszkieta L, Malireddi RKS, Karki R, Christgen S, Bomme P, Vogel P, Beau R, Mellado E, Ibrahim-Granet O, Henrissat B, Kalathur RC, Robinson C, Latgé JP, Kanneganti TD (2020) Galactosaminogalactan activates the inflammasome to provide host protection. Nature 588(7839):688–692. https://doi.org/10.1038/s41586-020-2996-z
Burm SM, Peferoen LA, Zuiderwijk-Sick EA, Haanstra KG, ’t Hart BA, van der Valk P, Amor S, Bauer J, Bajramovic JJ, (2016) Expression of IL-1β in rhesus EAE and MS lesions is mainly induced in the CNS itself. J Neuroinflammation 13(1):138. https://doi.org/10.1186/s12974-016-0605-8
Cao G, Wang Q, Huang W, Tong J, Ye D, He Y, Liu Z, Tang X, Cheng H, Wen Q, Li D, Chau HT, Wen Y, Zhong H, Meng Z, Liu H, Wu Z, Zhao L, Flavell RA, Zhou H, Xu A, Yang H, Yin Z (2017) Long-term consumption of caffeine-free high sucrose cola beverages aggravates the pathogenesis of EAE in mice. Cell Discov 3:17020. https://doi.org/10.1038/celldisc.2017.20
Chen Y, Sun JX, Chen WK, Wu GC, Wang YQ, Zhu KY, Wang J (2019) miR-124/VAMP3 is a novel therapeutic target for mitigation of surgical trauma-induced microglial activation. Signal Transduct Target Ther 4:27. https://doi.org/10.1038/s41392-019-0061-x
Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21(3):248–255. https://doi.org/10.1038/nm.3806
Comi G, Radaelli M, Soelberg Sørensen P (2017) Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 389(10076):1347–1356. https://doi.org/10.1016/s0140-6736(16)32388-1
Contarini G, Franceschini D, Facci L, Barbierato M, Giusti P, Zusso M (2019) A co-ultramicronized palmitoylethanolamide/luteolin composite mitigates clinical score and disease-relevant molecular markers in a mouse model of experimental autoimmune encephalomyelitis. J Neuroinflammation 16(1):126. https://doi.org/10.1186/s12974-019-1514-4
Ferrante A, Boussadia Z, Borreca A, Mallozzi C, Pedini G, Pacini L, Pezzola A, Armida M, Vincenzi F, Varani K, Bagni C, Popoli P, Martire A (2021) Adenosine A receptor inhibition reduces synaptic and cognitive hippocampal alterations in Fmr1 KO mice. Transl Psychiatry 11(1):112. https://doi.org/10.1038/s41398-021-01238-5
Fetisova E, Chernyak B, Korshunova G, Muntyan M, Skulachev V (2017) Mitochondria-targeted Antioxidants as a Prospective Therapeutic Strategy for Multiple Sclerosis. Curr Med Chem 24(19):2086–2114. https://doi.org/10.2174/0929867324666170316114452
Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, Mahad D, Bradl M, van Horssen J, Lassmann H (2012) NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 135:886–899. https://doi.org/10.1093/brain/aws012
Glatigny S, Bettelli E (2018) Experimental Autoimmune Encephalomyelitis (EAE) as Animal Models of Multiple Sclerosis (MS). Cold Spring Harb Perspect Med 8(11). https://doi.org/10.1101/cshperspect.a028977
Hagenow S, Affini A, Pioli EY, Hinz S, Zhao Y, Porras G, Namasivayam V, Müller CE, Lin JS, Bezard E, Stark H (2021) Adenosine AR/AR Antagonists Enabling Additional HR Antagonism for the Treatment of Parkinson’s Disease. J Med Chem 64(12):8246–8262. https://doi.org/10.1021/acs.jmedchem.0c00914
Han X, Sun S, Sun Y, Song Q, Zhu J, Song N, Chen M, Sun T, Xia M, Ding J, Lu M, Yao H, Hu G (2019) Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: implications for Parkinson disease. Autophagy 15(11):1860–1881. https://doi.org/10.1080/15548627.2019.1596481
Harun-Or-Rashid M, Inman DM (2018) Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma. J Neuroinflammation 15(1):313. https://doi.org/10.1186/s12974-018-1346-7
He T, Li W, Song Y, Li Z, Tang Y, Zhang Z, Yang GY (2020) Sestrin2 regulates microglia polarization through mTOR-mediated autophagic flux to attenuate inflammation during experimental brain ischemia. J Neuroinflammation 17(1):329. https://doi.org/10.1186/s12974-020-01987-y
Hedström AK, Mowry EM, Gianfrancesco MA, Shao X, Schaefer CA, Shen L, Olsson T, Barcellos LF, Alfredsson L (2016) High consumption of coffee is associated with decreased multiple sclerosis risk; results from two independent studies. J Neurol Neurosurg Psychiatry 87(5):454–460. https://doi.org/10.1136/jnnp-2015-312176
Huang SK, Pandey A, Tran DP, Villanueva NL, Kitao A, Sunahara RK, Prosser RS (2021) Delineating the conformational landscape of the adenosine A receptor during G protein coupling. Cell 184(7):1884–94.e14. https://doi.org/10.1016/j.cell.2021.02.041
Ingwersen J, Wingerath B, Graf J, Lepka K, Hofrichter M, Schröter F, Wedekind F, Bauer A, Schrader J, Hartung HP, Prozorovski T, Aktas O (2016) Dual roles of the adenosine A2a receptor in autoimmune neuroinflammation. J Neuroinflammation 13:48. https://doi.org/10.1186/s12974-016-0512-z
Jha S, Srivastava SY, Brickey WJ, Iocca H, Toews A, Morrison JP, Chen VS, Gris D, Matsushima GK, Ting JP (2010) The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J Neurosci 30(47):15811–15820. https://doi.org/10.1523/jneurosci.4088-10.2010
Kang CH, Jayasooriya RG, Dilshara MG, Choi YH, Jeong YK, Kim ND, Kim GY (2012) Caffeine suppresses lipopolysaccharide-stimulated BV2 microglial cells by suppressing Akt-mediated NF-κB activation and ERK phosphorylation. Food Chem Toxicol 50(12):4270–4276. https://doi.org/10.1016/j.fct.2012.08.041
Katayama H, Hama H, Nagasawa K, Kurokawa H, Sugiyama M, Ando R, Funata M, Yoshida N, Homma M, Nishimura T, Takahashi M, Ishida Y, Hioki H, Tsujihata Y, Miyawaki A (2020) Visualizing and Modulating Mitophagy for Therapeutic Studies of Neurodegeneration. Cell 181(5):1176-1187.e16. https://doi.org/10.1016/j.cell.2020.04.025
Kleerekooper I, Petzold A, Trip SA (2020) Anterior visual system imaging to investigate energy failure in multiple sclerosis. Brain 143(7):1999–2008. https://doi.org/10.1093/brain/awaa049
Kumar S, Jain A, Choi SW, Duarte P, da Silva G, Allers L, Mudd MH, Peters RS, Anonsen JH, Rusten TE, Lazarou M, Deretic V (2020) Mammalian Atg8-family proteins are upstream regulators of the lysosomalsystem by controlling MTOR and TFEB. Autophagy 16(12):2305–2306. https://doi.org/10.1080/15548627.2020.1837423
Labuschagne CF, Cheung EC, Blagih J, Domart MC, Vousden KH (2019) Cell Clustering Promotes a Metabolic Switch that Supports Metastatic Colonization. Cell Metab 30(4):720-734.e5. https://doi.org/10.1016/j.cmet.2019.07.014
Li L, Sun S, Tan L, Wang Y, Wang L, Zhang Z, Zhang L (2019a) Polystyrene Nanoparticles Reduced ROS and Inhibited Ferroptosis by Triggering Lysosome Stress and TFEB Nucleus Translocation in a Size-Dependent Manner. Nano Lett 19(11):7781–7792. https://doi.org/10.1021/acs.nanolett.9b02795
Li Y, Zhou D, Ren Y, Zhang Z, Guo X, Ma M, Xue Z, Lv J, Liu H, Xi Q, Jia L, Zhang L, Liu Y, Zhang Q, Yan J, Da Y, Gao F, Yue J, Yao Z, Zhang R (2019b) Mir223 restrains autophagy and promotes CNS inflammation by targeting ATG16L1. Autophagy 15(3):478–492. https://doi.org/10.1080/15548627.2018.1522467
Li YF, Ouyang SH, Tu LF, Wang X, Yuan WL, Wang GE, Wu YP, Duan WJ, Yu HM, Fang ZZ, Kurihara H, Zhang Y, He RR (2018) Caffeine Protects Skin from Oxidative Stress-Induced Senescence through the Activation of Autophagy. Theranostics 8(20):5713–5730. https://doi.org/10.7150/thno.28778
Lian H, Litvinchuk A, Chiang AC, Aithmitti N, Jankowsky JL, Zheng H (2016) Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer’s Disease. J Neurosci 36(2):577–589. https://doi.org/10.1523/jneurosci.2117-15.2016
Liang P, Le W (2015) Role of autophagy in the pathogenesis of multiple sclerosis. Neurosci Bull 31(4):435–444. https://doi.org/10.1007/s12264-015-1545-5
Liston A, Masters SL (2017) Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat Rev Immunol 17(3):208–214. https://doi.org/10.1038/nri.2016.151
Liu P, Huang G, Wei T, Gao J, Huang C, Sun M, Zhu L (1864) Shen W (2018) Sirtuin 3-induced macrophage autophagy in regulating NLRP3 inflammasome activation. Biochim Biophys Acta Mol Basis Dis 3:764–777. https://doi.org/10.1016/j.bbadis.2017.12.027
Lopes CR, Cunha RA, Agostinho P (2021) Astrocytes and Adenosine A Receptors: Active Players in Alzheimer’s Disease. Front Neurosci 15:666710. https://doi.org/10.3389/fnins.2021.666710
Luan Y, Ren X, Zheng W, Zeng Z, Guo Y, Hou Z, Guo W, Chen X, Li F, Chen JF (2018) Chronic Caffeine Treatment Protects Against α-Synucleinopathy by Reestablishing Autophagy Activity in the Mouse Striatum. Front Neurosci 12:301. https://doi.org/10.3389/fnins.2018.00301
Lubetzki C, Zalc B, Williams A, Stadelmann C, Stankoff B (2020) Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol 19(8):678–688. https://doi.org/10.1016/s1474-4422(20)30140-x
Malhotra S, Costa C, Eixarch H, Keller CW, Amman L, Martínez-Banaclocha H, Midaglia L, Sarró E, Machín-Díaz I, Villar LM, Triviño JC, Oliver-Martos B, Parladé LN, Calvo-Barreiro L, Matesanz F, Vandenbroeck K, Urcelay E, Martínez-Ginés ML, Tejeda-Velarde A, Fissolo N, Castilló J, Sanchez A, Robertson AAB, Clemente D, Prinz M, Pelegrin P, Lünemann JD, Espejo C, Montalban X, Comabella M (2020) NLRP3 inflammasome as prognostic factor and therapeutic target in primary progressive multiple sclerosis patients. Brain 143(5):1414–1430. https://doi.org/10.1093/brain/awaa084
Mao ZF, Ouyang SH, Zhang QY, Wu YP, Wang GE, Tu LF, Luo Z, Li WX, Kurihara H, Li YF, He RR (2020) New insights into the effects of caffeine on adult hippocampal neurogenesis in stressed mice: Inhibition of CORT-induced microglia activation. FASEB J 34(8):10998–11014. https://doi.org/10.1096/fj.202000146RR
Mijaljica D, Klionsky DJ (2020) Autophagy/virophagy: a “disposal strategy” to combat COVID-19. Autophagy 16(12):2271–2272. https://doi.org/10.1080/15548627.2020.1782022
Mizushima N, Levine B (2020) Autophagy in Human Diseases. N Engl J Med 383(16):1564–1576. https://doi.org/10.1056/NEJMra2022774
Nicoletti F, Di Marco R, Mangano K, Patti F, Reggio E, Nicoletti A, Bendtzen K, Reggio A (2001) Increased serum levels of interleukin-18 in patients with multiple sclerosis. Neurology 57(2):342–344. https://doi.org/10.1212/wnl.57.2.342
Okuda Y, Sakoda S, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T (1999) IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glucoprotein 35–55 induced experimental autoimmune encephalomyelitis. J Neuroimmunol 101(2):188–196. https://doi.org/10.1016/s0165-5728(99)00139-3
Orr AG, Orr AL, Li XJ, Gross RE, Traynelis SF (2009) Adenosine A(2A) receptor mediates microglial process retraction. Nat Neurosci 12(7):872–878. https://doi.org/10.1038/nn.2341
Pang Y, Dai X, Roller A, Carter K, Paul I, Bhatt AJ, Lin RC, Fan LW (2016) Early Postnatal Lipopolysaccharide Exposure Leads to Enhanced Neurogenesis and Impaired Communicative Functions in Rats. PLoS One 11(10):e0164403. https://doi.org/10.1371/journal.pone.0164403
Prinz M, Jung S, Priller J (2019) Microglia Biology: One Century of Evolving Concepts. Cell 179(2):292–311. https://doi.org/10.1016/j.cell.2019.08.053
Rossi S, Studer V, Motta C, Germani G, Macchiarulo G, Buttari F, Mancino R, Castelli M, De Chiara V, Weiss S, Martino G, Furlan R, Centonze D (2014) Cerebrospinal fluid detection of interleukin-1β in phase of remission predicts disease progression in multiple sclerosis. J Neuroinflammation 11:32. https://doi.org/10.1186/1742-2094-11-32
Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH (2012) Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol 13(3):255–263. https://doi.org/10.1038/ni.2215
Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306(5698):990–995. https://doi.org/10.1126/science.1099993
Sinha RA, Farah BL, Singh BK, Siddique MM, Li Y, Wu Y, Ilkayeva OR, Gooding J, Ching J, Zhou J, Martinez L, Xie S, Bay BH, Summers SA, Newgard CB, Yen PM (2014) Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology 59(4):1366–1380. https://doi.org/10.1002/hep.26667
Song KY, Guo XM, Wang HQ, Zhang L, Huang SY, Huo YC, Zhang G, Feng JZ, Zhang RR, Ma Y, Hu QZ, Qin XY (2020) MBNL1 reverses the proliferation defect of skeletal muscle satellite cells in myotonic dystrophy type 1 by inhibiting autophagy via the mTOR pathway. Cell Death Dis 11(7):545. https://doi.org/10.1038/s41419-020-02756-8
Stromnes IM, Goverman JM (2006) Active induction of experimental allergic encephalomyelitis. Nat Protoc 1(4):1810–1819. https://doi.org/10.1038/nprot.2006.285
Sun D, Yu Z, Fang X, Liu M, Pu Y, Shao Q, Wang D, Zhao X, Huang A, Xiang Z, Zhao C, Franklin RJ, Cao L, He C (2017) LncRNA GAS5 inhibits microglial M2 polarization and exacerbates demyelination. EMBO Rep 18(10):1801–1816. https://doi.org/10.15252/embr.201643668
Sun Z, Zeng B, Liu D, Zhao Q, Wang J, Rosie Xing H (2020) S100A8 transported by SEC23A inhibits metastatic colonization via autocrine activation of autophagy. Cell Death Dis 11(8):650. https://doi.org/10.1038/s41419-020-02835-w
Thompson A, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O (2018) Multiple sclerosis. Lancet 391(10130):1622–1636. https://doi.org/10.1016/s0140-6736(18)30481-1
van Dam R, Hu FB, Willett WC (2020) Coffee, Caffeine, and Health. N Engl J Med 383(4):369–378. https://doi.org/10.1056/NEJMra1816604
Voet S, McGuire C, Hagemeyer N, Martens A, Schroeder A, Wieghofer P, Daems C, Staszewski O, Vande Walle L, Jordao MJC, Sze M, Vikkula HK, Demeestere D, Van Imschoot G, Scott CL, Hoste E, Gonçalves A, Guilliams M, Lippens S, Libert C, Vandenbroucke RE, Kim KW, Jung S, Callaerts-Vegh Z, Callaerts P, de Wit J, Lamkanfi M, Prinz M, van Loo G (2018) A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat Commun 9(1):2036. https://doi.org/10.1038/s41467-018-04376-5
Voet S, Prinz M, van Loo G (2019) Microglia in Central Nervous System Inflammation and Multiple Sclerosis Pathology. Trends Mol Med 25(2):112–123. https://doi.org/10.1016/j.molmed.2018.11.005
Wang L, Hauenstein AV (2020) The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol Aspects Med 76:100889. https://doi.org/10.1016/j.mam.2020.100889
Wolf S, Boddeke H, Kettenmann H (2017) Microglia in Physiology and Disease. Annu Rev Physiol 79:619–643. https://doi.org/10.1146/annurev-physiol-022516-034406
Xu D, Zhao H, Jin M, Zhu H, Shan B, Geng J, Dziedzic SA, Amin P, Mifflin L, Naito MG, Najafov A, Xing J, Yan L, Liu J, Qin Y, Hu X, Wang H, Zhang M, Manuel VJ, Tan L, He Z, Sun ZJ, Lee VMY, Wagner G, Yuan J (2020) Modulating TRADD to restore cellular homeostasis and inhibit apoptosis. Nature 587(7832):133–138. https://doi.org/10.1038/s41586-020-2757-z
Xue Z, Zhang Z, Liu H, Li W, Guo X, Zhang Z, Liu Y, Jia L, Li Y, Ren Y, Yang H, Zhang L, Zhang Q, Da Y, Hao J, Yao Z, Zhang R (2019) lincRNA-Cox2 regulates NLRP3 inflammasome and autophagy mediated neuroinflammation. Cell Death Differ 26(1):130–145. https://doi.org/10.1038/s41418-018-0105-8
Yan R, Zhang J, Park HJ, Park ES, Oh S, Zheng H, Junn E, Voronkov M, Stock JB, Mouradian MM (2018) Synergistic neuroprotection by coffee components eicosanoyl-5-hydroxytryptamide and caffeine in models of Parkinson’s disease and DLB. Proc Natl Acad Sci U S A 115(51):E12053–E12062. https://doi.org/10.1073/pnas.1813365115
Ye X, Zhu M, Che X, Wang H, Liang XJ, Wu C, Xue X, Yang J (2020) Lipopolysaccharide induces neuroinflammation in microglia by activating the MTOR pathway and downregulating Vps34 to inhibit autophagosome formation. J Neuroinflammation 17(1):18. https://doi.org/10.1186/s12974-019-1644-8
Yuan B, Shen H, Lin L, Su T, Zhong L, Yang Z (2017) Autophagy Promotes Microglia Activation Through Beclin-1-Atg5 Pathway in Intracerebral Hemorrhage. Mol Neurobiol 54(1):115–124. https://doi.org/10.1007/s12035-015-9642-z
Zhang Y, Han JJ, Liang XY, Zhao L, Zhang F, Rasouli J, Wang ZZ, Zhang GX, Li X (2018) miR-23b Suppresses Leukocyte Migration and Pathogenesis of Experimental Autoimmune Encephalomyelitis by Targeting CCL7. Mol Ther 26(2):582–592. https://doi.org/10.1016/j.ymthe.2017.11.013
Zhao J, Hu B, Xiao H, Yang Q, Cao Q, Li X, Zhang Q, Ji A, Song S (2021) Fucoidan reduces lipid accumulation by promoting foam cell autophagy via TFEB. Carbohydr Polym 268:118247. https://doi.org/10.1016/j.carbpol.2021.118247
Acknowledgements
We thank the Laboratory of Pharmacology, Chongqing Medical University for sharing the cell lines in this study.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81771275), the National Natural Science Foundation of China Youth Fund Project (Grant No. 81701191).
Author information
Authors and Affiliations
Contributions
Hui-Qi Wang and Kai-Yi Song: study design, experimental operations, data sorting, statistical analysis, and manuscript preparation; Jin-Zhou Feng and Si-Yuan Huang: study design and experimental operations; Xiu-Ming Guo, Lei Zhang, and Gang Zhang: data sorting and experimental operations; Ying-Chao Huo, Rong-Rong Zhang, Yue Ma, and Qing-Zhe Hu: data sorting, statistical analysis, and experimental operations; Xin-Yue Qin: study design, data analysis, and final manuscript preparation and revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Data in this study were collected from animals. This study was in line with the requirements of the Ethics Committee for Animal Experimentation of Chongqing Medical University.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, HQ., Song, KY., Feng, JZ. et al. Caffeine Inhibits Activation of the NLRP3 Inflammasome via Autophagy to Attenuate Microglia-Mediated Neuroinflammation in Experimental Autoimmune Encephalomyelitis. J Mol Neurosci 72, 97–112 (2022). https://doi.org/10.1007/s12031-021-01894-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-021-01894-8