Abstract
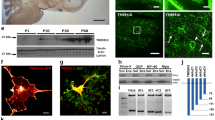
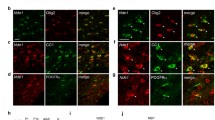
Oligodendrocytes, the myelin-forming cells of the central nervous system, play important roles in brain development and maintenance. Activity-dependent neuroprotective protein (ADNP), an early marker essential for brain formation, interacts with microtubule end-binding proteins (EB1, EB2, and EB3). EB1 and EB3 are highly expressed in neurons (axons and dendritic spines, respectively) and EB1 enhancement of neurite outgrowth is attenuated by EB2. ADNP/EB presence in oligodendrocytes has not been studied so far. Here, we measured messenger RNA (mRNA) levels of ADNP and EB1–EB3 in rat brain oligodendrocytes during culture maturation and in rat brains during development (1, 35, and 75 days) in comparison with rat astrocytes, dorsal root ganglion (DRG) neurons, and the oligodendroglia cell lines (OLN-93 cell line, not expressing the microtubule-associated protein (MAP) tau, and OLN-93 cells stably transfected to express various forms of tau). Results showed that all transcripts studied were expressed in oligodendrocytes. ADNP and EB2 mRNA transcript content peaked at the time of oligodendrocyte maturation (5 days in vitro) and was highest in newborn rat brains compared with mature brains. ADNP2 (the only family member of ADNP), and EB1, although expressed in lower quantities, essentially paralleled ADNP and EB2 expression patterns, respectively. EB3 mRNA, peaking upon oligodendrocyte maturation, showed an apparent second peak of expression (10 days in vitro) and increased in the mature rat brain compared with the newborn brain. DRG cells expressed the highest levels of EB3, when compared with oligodendrocyte precursors and with astrocytes but not when compared with mature oligodendrocytes. Mature oligodendrocytes and oligodendrocyte precursors expressed ~30–40-fold more EB2 vs. EB3, and ~4–7-fold vs. ADNP. DRGs expressed ~5-fold more EB2 vs. EB3 and astrocytes showed an in-between (~20-fold) ratio. Only DRGs expressed similar EB1 and EB3 transcript levels, contrasting with oligodendrocyte and astrocytes (~10–30-fold more EB1). Astrocytes expressed more ADNP than DRGs and oligodendrocyte precursor cells (~2-fold) but not compared with mature oligodendrocytes. EB1 and EB3 were previously found to be associated with tau. Immortalized oligodendrocytes showed an intermediate phenotype of mRNA expression compared with oligodendrocyte precursor cells and mature oligodendrocytes with tau transfection reducing overall ADNP and EB expression. In summary, ADNPs and EBs are highly expressed in oligodendrocytes suggesting an impact on myelin formation in health and disease.



Similar content being viewed by others
References
Arens J, Duong TT, Dehmelt L (2013) A morphometric screen identifies specific roles for microtubule-regulating genes in neuronal development of P19 stem cells. PLoS One 8:e79796
Bartzokis G, Lu PH, Geschwind DH, Tingus K, Huang D, Mendez MF, Edwards N, Mintz J (2007) Apolipoprotein E affects both myelin breakdown and cognition: implications for age-related trajectories of decline into dementia. Biol Psychiatry 62:1380–1387
Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, Bassan H, Blat C, Gibney G, Glazner G, Brenneman DE, Gozes I (1999) Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem 72:1283–1293
Bauer NG, Richter-Landsberg C (2006) The dynamic instability of microtubules is required for aggresome formation in oligodendroglial cells after proteolytic stress. J Mol Neurosci 29:153–168
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927
Braitch M, Kawabe K, Nyirenda M, Gilles LJ, Robins RA, Gran B, Murphy S, Showe L, Constantinescu CS (2009) Expression of activity-dependent neuroprotective protein in the immune system: possible functions and relevance to multiple sclerosis. Neuroimmunomodulation 17:120–125
Chambers RA, Krystal JH, Self DW (2001) A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry 50:71–83
De Groot CO, Jelesarov I, Damberger FF, Bjelic S, Scharer MA, Bhavesh NS, Grigoriev I, Buey RM, Wuthrich K, Capitani G, Akhmanova A, Steinmetz MO (2010) Molecular insights into mammalian end-binding protein heterodimerization. J Biol Chem 285:5802–5814
Dresner E, Agam G, Gozes I (2011) Activity-dependent neuroprotective protein (ADNP) expression level is correlated with the expression of the sister protein ADNP2: deregulation in schizophrenia. Eur Neuropsychopharmacol 21:355–361
Dresner E, Malishkevich A, Arviv C, Leibman Barak S, Alon S, Ofir R, Gothilf Y, Gozes I (2012) Novel evolutionary-conserved role for the activity-dependent neuroprotective protein (ADNP) family that is important for erythropoiesis. J Biol Chem 287:40173–40185
Fields RD (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31:361–370
Frohman EM, Racke MK, Raine CS (2006) Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med 354:942–955
Furman S, Steingart RA, Mandel S, Hauser JM, Brenneman DE, Gozes I (2004) Subcellular localization and secretion of activity-dependent neuroprotective protein in astrocytes. Neuron Glia Biol 1:193–199
Gamsiz ED, Sciarra LN, Maguire AM, Pescosolido MF, van Dyck LI, Morrow EM (2015) Discovery of rare mutations in autism: elucidating neurodevelopmental mechanisms. Neurotherapeutics : J Am Soc Exp NeuroTherapeutics 12:553–571
Geraldo S, Khanzada UK, Parsons M, Chilton JK, Gordon-Weeks PR (2008) Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nat Cell Biol 10:1181–1189
Goedert M, Crowther RA, Garner CC (1991) Molecular characterization of microtubule-associated proteins tau and MAP2. Trends Neurosci 14:193–199
Goldbaum O, Oppermann M, Handschuh M, Dabir D, Zhang B, Forman MS, Trojanowski JQ, Lee VM, Richter-Landsberg C (2003) Proteasome inhibition stabilizes tau inclusions in oligodendroglial cells that occur after treatment with okadaic acid. J Neurosci 23:8872–8880
Goldbaum O, Richter-Landsberg C (2004) Proteolytic stress causes heat shock protein induction, tau ubiquitination, and the recruitment of ubiquitin to tau-positive aggregates in oligodendrocytes in culture. J Neurosci 24:5748–5757
Goldbaum O, Riedel M, Stahnke T, Richter-Landsberg C (2009) The small heat shock protein HSP25 protects astrocytes against stress induced by proteasomal inhibition. Glia 57:1566–1577
Gorath M, Stahnke T, Mronga T, Goldbaum O, Richter-Landsberg C (2001) Developmental changes of tau protein and mRNA in cultured rat brain oligodendrocytes. Glia 36:89–101
Gozes I (2015) The cytoskeleton as a drug target for neuroprotection: the case of the autism-mutated ADNP. Biological Chemistry
Gozes I, Richter-Landsberg C (1978) Identification of tubulin associated with rat brain myelin. FEBS Lett 95:169–172
Gozes I, Helsmoortel C, Vandeweyer G, Van der Aa N, Kooy F, Sermone SB (2015) The compassionate side of neuroscience: Tony Sermone’s undiagnosed genetic journey-ADNP mutation. J Mol Neurosci
Gu C, Zhou W, Puthenveedu MA, Xu M, Jan YN, Jan LY (2006) The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron 52:803–816
Helsmoortel C, Vulto-van Silfhout AT, Coe BP, Vandeweyer G, Rooms L, van den Ende J, Schuurs-Hoeijmakers JH, Marcelis CL, Willemsen MH, Vissers LE, Yntema HG, Bakshi M, Wilson M, Witherspoon KT, Malmgren H, Nordgren A, Anneren G, Fichera M, Bosco P, Romano C, de Vries BB, Kleefstra T, Kooy RF, Eichler EE, Van der Aa N (2014) A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet 46:380–384
Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, Grigoriev I, van Rijssel FJ, Buey RM, Lawera A, Jelesarov I, Winkler FK, Wuthrich K, Akhmanova A, Steinmetz MO (2009) An EB1-binding motif acts as a microtubule tip localization signal. Cell 138:366–376
Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J, Krugers H, Defilippi P, Akhmanova A, Hoogenraad CC (2009) Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 61:85–100
Kalbfuss B, Mabon SA, Misteli T (2001) Correction of alternative splicing of tau in frontotemporal dementia and parkinsonism linked to chromosome 17. J Biol Chem 276:42986–42993
Komarova Y, Lansbergen G, Galjart N, Grosveld F, Borisy GG, Akhmanova A (2005) EB1 and EB3 control CLIP dissociation from the ends of growing microtubules. Mol Biol Cell 16:5334–5345
Kushnir M, Dresner E, Mandel S, Gozes I (2008) Silencing of the ADNP-family member, ADNP2, results in changes in cellular viability under oxidative stress. J Neurochem 105:537–545
Magen I, Gozes I (2013) Microtubule-stabilizing peptides and small molecules protecting axonal transport and brain function: focus on davunetide (NAP). Neuropeptides 47:489–495
Magen I, Gozes I (2014) Davunetide: peptide therapeutic in neurological disorders. Curr Med Chem
Malishkevich A, Amram N, Hacohen-Kleiman G, Magen I, Giladi E, Gozes I (2015) Activity-dependent neuroprotective protein (ADNP) exhibits striking sexual dichotomy impacting on autistic and Alzheimer’s pathologies. Transl Psychiatry 5:e501
Mandel S, Gozes I (2007) Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J Biol Chem 282:34448–34456
Mandel S, Rechavi G, Gozes I (2007) Activity-dependent neuroprotective protein (ADNP) differentially interacts with chromatin to regulate genes essential for embryogenesis. Dev Biol 303:814–824
Mandel S, Spivak-Pohis I, Gozes I (2008) ADNP differential nucleus/cytoplasm localization in neurons suggests multiple roles in neuronal differentiation and maintenance. J Mol Neurosci 35:127–141
Merenlender-Wagner A, Shemer Z, Touloumi O, Lagoudaki R, Giladi E, Andrieux A, Grigoriadis NC, Gozes I (2014) New horizons in schizophrenia treatment: autophagy protection is coupled with behavioral improvements in a mouse model of schizophrenia. Autophagy 0
Merenlender-Wagner A, Malishkevich A, Shemer Z, Udawela M, Gibbons A, Scarr E, Dean B, Levine J, Agam G, Gozes I (2015) Autophagy has a key role in the pathophysiology of schizophrenia. Mol Psychiatry 20:126–132
Muller R, Heinrich M, Heck S, Blohm D, Richter-Landsberg C (1997) Expression of microtubule-associated proteins MAP2 and tau in cultured rat brain oligodendrocytes. Cell Tissue Res 288:239–249
Nakagawa H, Koyama K, Murata Y, Morito M, Akiyama T, Nakamura Y (2000) EB3, a novel member of the EB1 family preferentially expressed in the central nervous system, binds to a CNS-specific APC homologue. Oncogene 19:210–216
Noack M, Leyk J, Richter-Landsberg C (2014) HDAC6 inhibition results in tau acetylation and modulates tau phosphorylation and degradation in oligodendrocytes. Glia 62:535–547
Noll E, Miller RH (1993) Oligodendrocyte precursors originate at the ventral ventricular zone dorsal to the ventral midline region in the embryonic rat spinal cord. Development 118:563–573
Oksenberg JR, Baranzini SE, Sawcer S, Hauser SL (2008) The genetics of multiple sclerosis: SNPs to pathways to pathogenesis. Nat Rev Genet 9:516–526
Ou-Yang MH, Xu F, Liao MC, Davis J, Robinson JK, Van Nostrand WE (2015) The N-terminal region of myelin basic protein reduces fibrillar amyloid-beta deposition in Tg-5xFAD mice. Neurobiol Aging 36:801–811
Oz S, Kapitansky O, Ivashco-Pachima Y, Malishkevich A, Giladi E, Skalka N, Rosin-Arbesfeld R, Mittelman L, Segev O, Hirsch JA, Gozes I (2014) The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol Psychiatry 19:1115–1124
Pescosolido MF, Schwede M, Johnson Harrison A, Schmidt M, Gamsiz ED, Chen WS, Donahue JP, Shur N, Jerskey BA, Phornphutkul C, Morrow EM (2014) Expansion of the clinical phenotype associated with mutations in activity-dependent neuroprotective protein. J Med Genet 51:587–589
Pinhasov A, Mandel S, Torchinsky A, Giladi E, Pittel Z, Goldsweig AM, Servoss SJ, Brenneman DE, Gozes I (2003) Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res Dev Brain Res 144:83–90
Quintana FJ, Zaltzman R, Fernandez-Montesinos R, Herrera JL, Gozes I, Cohen IR, Pozo D (2006) NAP, a peptide derived from the activity-dependent neuroprotective protein, modulates macrophage function. Ann N Y Acad Sci 1070:500–506
Rajendran L, Bali J, Barr MM, Court FA, Kramer-Albers EM, Picou F, Raposo G, van der Vos KE, van Niel G, Wang J, Breakefield XO (2014) Emerging roles of extracellular vesicles in the nervous system. J Neurosci 34:15482–15489
Richter-Landsberg C, Gorath M (1999) Developmental regulation of alternatively spliced isoforms of mRNA encoding MAP2 and tau in rat brain oligodendrocytes during culture maturation. J Neurosci Res 56:259–270
Richter-Landsberg C, Heinrich M (1996) OLN-93: a new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J Neurosci Res 45:161–173
Richter-Landsberg C, Leyk J (2013) Inclusion body formation, macroautophagy, and the role of HDAC6 in neurodegeneration. Acta Neuropathol 126:793–807
Sayas CL, Tortosa E, Bollati F, Ramirez-Rios S, Arnal I, Avila J (2015) Tau regulates the localization and function of End-binding proteins 1 and 3 in developing neuronal cells. J Neurochem 133:653–667
Schirer Y, Malishkevich A, Ophir Y, Lewis J, Giladi E, Gozes I (2014) Novel marker for the onset of frontotemporal dementia: early increase in activity-dependent neuroprotective protein (ADNP) in the face of Tau mutation. PLoS One 9:e87383
Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL, Gattaz WF, Bogerts B, Schmitz C, Falkai P (2009) Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol 117:395–407
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Seiberlich V, Bauer NG, Schwarz L, Ffrench-Constant C, Goldbaum O, Richter-Landsberg C (2015) Downregulation of the microtubule associated protein Tau impairs process outgrowth and myelin basic protein mRNA transport in oligodendrocytes. Glia
Sen I, Veprintsev D, Akhmanova A, Steinmetz MO (2013) End binding proteins are obligatory dimers. PLoS One 8:e74448
Sokolowska P, Passemard S, Mok A, Schwendimann L, Gozes I, Gressens P (2011) Neuroprotective effects of NAP against excitotoxic brain damage in the newborn mice: implications for cerebral palsy. Neuroscience 173:156–168
Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N (2003) Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J Neurosci 23:2655–2664
Su LK, Qi Y (2001) Characterization of human MAPRE genes and their proteins. Genomics 71:142–149
Vulih-Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z, Gozes I (2007) Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J Pharmacol Exp Ther 323:438–449
Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E, Bassan M, Wollman Y, Eyre HJ, Mulley JC, Brenneman DE, Gozes I (2001) Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem 276:708–714
Acknowledgments
Support was provided by the AMN Foundation, Israel Science Foundation and the Israeli Ministry for Science Technology and Space. Professor Illana Gozes is the incumbent of the Lily and Avraham Gildor Chair for the Investigation of Growth Factors, the Director of the Levie-Edersheim-Gitter Institute for Functional Brain Imaging, and the Dr. Diana and Zelman Elton (Elbaum) Laboratory for Molecular Neuroendocrinology at Tel Aviv University and a Humboldt Award Recipient, currently a fellow at Hanse-Wissenschaftskolleg (HWK), Germany. This study is in partial fulfillment for the Ph.D. requirement for Mrs. Anna Malishkevich at the Dr. Miriam and Sheldon G. Adelson Graduate School in Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malishkevich, A., Leyk, J., Goldbaum, O. et al. ADNP/ADNP2 expression in oligodendrocytes: implication for myelin-related neurodevelopment. J Mol Neurosci 57, 304–313 (2015). https://doi.org/10.1007/s12031-015-0640-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0640-4